Nucleic acid detection reagent kit for novel coronavirus 2019-nCoV
A 2019-ncov, coronavirus technology, applied in the field of biotechnology and molecular diagnosis, can solve the problems of lack of simultaneous detection of multiple genes, unfavorable virus detection and diagnosis, and large differences in reagent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Example 1 Detection limit test of novel coronavirus 2019-nCoV nucleic acid detection kit
[0119] Dilute the pseudovirus with the determined value as the initial sample to a concentration of 10 5 copies / ml, serially diluted to 10 4 、10 3 , 500, 250copies / ml, each concentration sample was added to the final concentration of 10 4 Copies / ml of the pseudovirus containing the amplified fragment of the internal standard is used as the sample to be tested, and the sensitivity of the 3-fold detection reagent is tested. The PCR reaction system comprises the combination of primers and probes shown in SEQ ID NO.: 1-12 of the present invention.
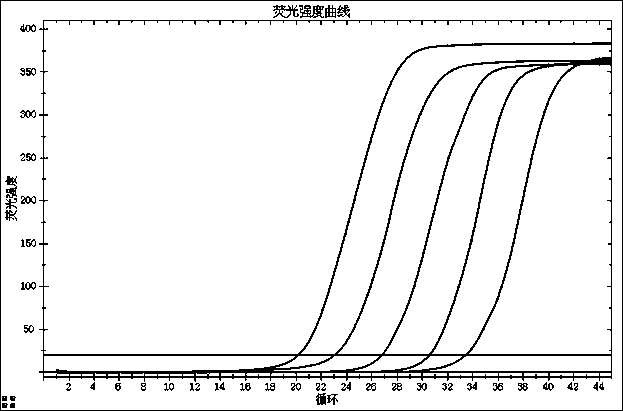
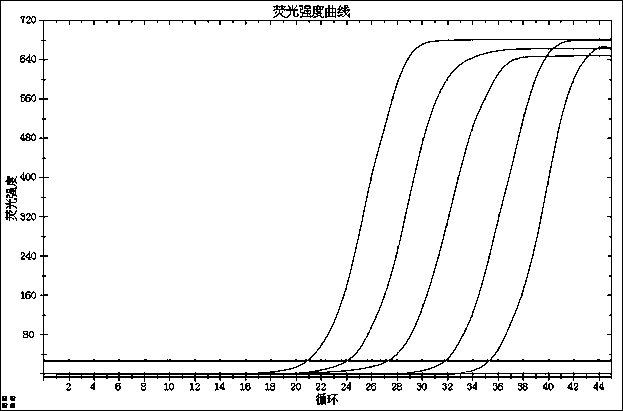
[0120] For test results, see Figure 1-4 :
[0121] figure 1 : N gene detection limit;
[0122] figure 2 : ORF1ab gene detection limit test;
[0123] image 3 : E gene detection limit test;
[0124] Figure 4 : internal standard test result.
[0125] The results showed that the lowest detectable concentration was 500copies / ...
Embodiment 2
[0126] Example 2 Specificity Test of New Coronavirus 2019-nCoV Nucleic Acid Detection Kit
[0127] Other pathogens that are similar to the 2019 new coronavirus species or cause similar symptoms (such as seasonal influenza A H1N1 virus, new type A H1N1 (2009) influenza virus, influenza A H3N2, H5N1, H7N9, influenza B Yamagata, Influenza B Victoria, RSV A, RSV B, parainfluenza virus, adenovirus, enterovirus, human metapneumovirus (human metapneumovirus), Epstein-Barr virus, measles virus, human giant Cytovirus, rotavirus, norovirus, mumps virus, varicella-zoster virus, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella, Bordetella pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, pyogenic Streptococcus, Klebsiella pneumoniae, Mycobacterium tuberculosis, Aspergillus fumigatus, Candida albicans, Candida glabrata, Cryptococcus neoformans, coronavirus (HKU1, OC43, NL63, 229E) and human genomic DNA as specific reference materials, Test the spec...
Embodiment 3
[0132] Example 3, Precision Test of Novel Coronavirus 2019-nCoV Nucleic Acid Detection Kit
[0133] N / ORF1ab / E gene pseudovirus was diluted to 10 5 and 10 3 Copies / ml was used as a precision reference product, each repeated 10 times, and the coefficient of variation of each concentration precision reference product was calculated.
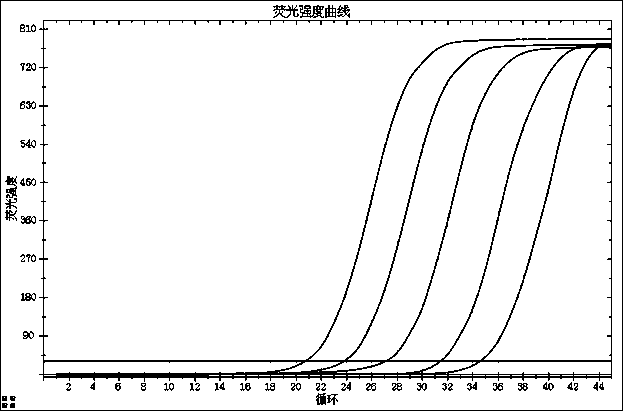
[0134] For test results, see Figure 7-9 :
[0135] Figure 7 . N gene precision test results;
[0136] Figure 8 . ORF1ab gene precision test results;
[0137] Figure 9 .E gene precision test results;
[0138] After testing, the coefficients of variation of the new coronavirus 2019-nCoV high-concentration and low-concentration precision reference products are 1.16% and 1.54% for the N gene, 1.16% and 1.54% for the ORF1ab gene, and 1.16% and 1.54% for the E gene; The coefficients of variation of precision reference products with different concentrations of detection targets were all less than 5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com