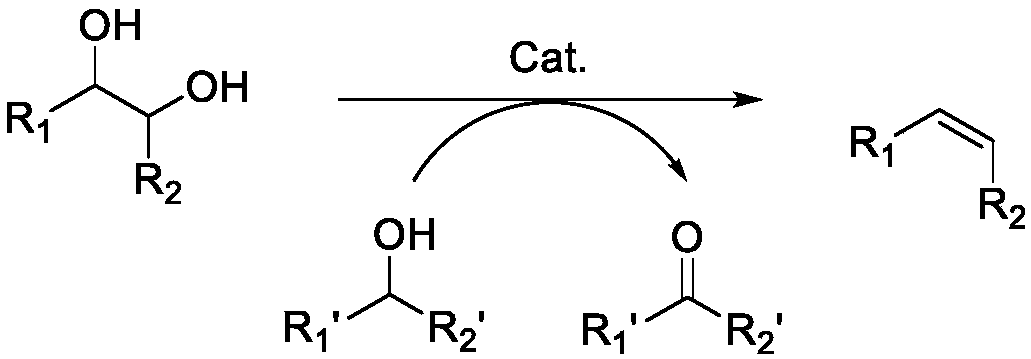
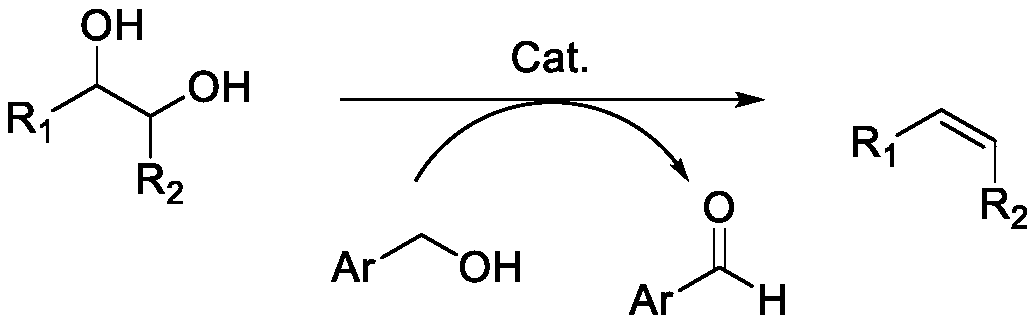
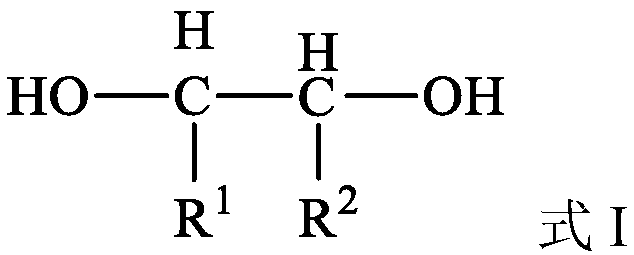
Method for preparing olefin by deoxidizing and dehydrating polyhydroxy compound by using alcohol as reducing agent
A polyhydroxy compound and reducing agent technology, which can be used in the preparation of organic compounds, hydrocarbon production from oxygen-containing organic compounds, chemical instruments and methods, etc. Wide range, high product selectivity, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 1-propanol as reducing agent
[0079] With 1-propanol reducing agent, put 0.25mmol substrate tartaric acid, 10mol% catalyst (relative to the substrate), 5mL 1-propanol in a 38mL pressure-resistant tube, and react in an oil bath at 200°C for 8h. After the reaction, it was placed in an ice-water bath to cool down to room temperature. A certain amount of reaction solution (1.5 ml) was taken, and an internal standard (1 ml, 0.005018 g / ml durene in acetonitrile solution) was added. GC-MS was used for qualitative analysis of the main product containing C=C, and GC (Agilent 7890A) was used for quantitative analysis. Qualitative analysis used GC-MS. Quantitative analysis showed that, depending on the catalysts (rhenium trioxide, ammonium perrhenate, molybdenum 8-hydroxyquinoline, respectively), the conversion rate of the substrate was >85%, and the yield of the product containing C=C was up to 60% -90%.
Embodiment 2
[0080] Embodiment 2 n-butanol is made reducing agent
[0081] With n-butanol reducing agent, put 0.25mmol substrate tartaric acid, 10mol% catalyst (relative to the substrate), 5mL n-butanol in a 38mL pressure-resistant tube, and react in an oil bath at 160°C for 12 hours, and the reaction is over Afterwards, it was cooled to room temperature in an ice-water bath. A certain amount of reaction solution (1.5 ml) was taken, and an internal standard (1 ml, 0.005018 g / ml durene in acetonitrile solution) was added. GC-MS was used for qualitative analysis of the main product containing C=C, and GC (Agilent 7890A) was used for quantitative analysis. Qualitative analysis used GC-MS. Quantitative analysis showed that, according to different catalysts (respectively, rhenium heptoxide, methyl rhenium trioxide, ammonium perrhenate, molybdenum 8-hydroxyquinoline), the conversion rate of the substrate was >85%, and the yield of the product containing C=C The rate reaches 60%-99%.
Embodiment 3
[0082] Example 3 1-pentanol as reducing agent
[0083] Put 0.25mmol substrate tartaric acid, 10mol% catalyst (relative to the substrate), and 5mL 1-pentanol in a 38mL pressure-resistant tube with a 1-pentanol reducing agent, and react in an oil bath at 180°C for 8 hours. When finished, cool to room temperature in an ice-water bath. A certain amount of reaction solution (1.5ml) was taken, and an internal standard (1ml, 0.005018g / ml durene in acetonitrile solution) was added. GC-MS was used for qualitative analysis of the main product containing C=C, and GC (Agilent 7890A) was used for quantitative analysis. Qualitative analysis used GC-MS. Quantitative analysis showed that, depending on the catalysts (dirhenium heptoxide, methylrhenium trioxide, ammonium perrhenate, molybdenum 8-hydroxyquinoline, respectively), the conversion of substrates was >80%, and the yield of products containing C=C The rate reaches 60%-90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com