A kind of polyphosphate kinase mutant and its application
A polyphosphate kinase and mutant technology, applied in the fields of genetic engineering and enzyme catalysis, can solve the problems of poor substrate tolerance, low enzyme activity, and high substrate inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Construction of wild-type polyphosphate kinase gene recombinant Escherichia coli
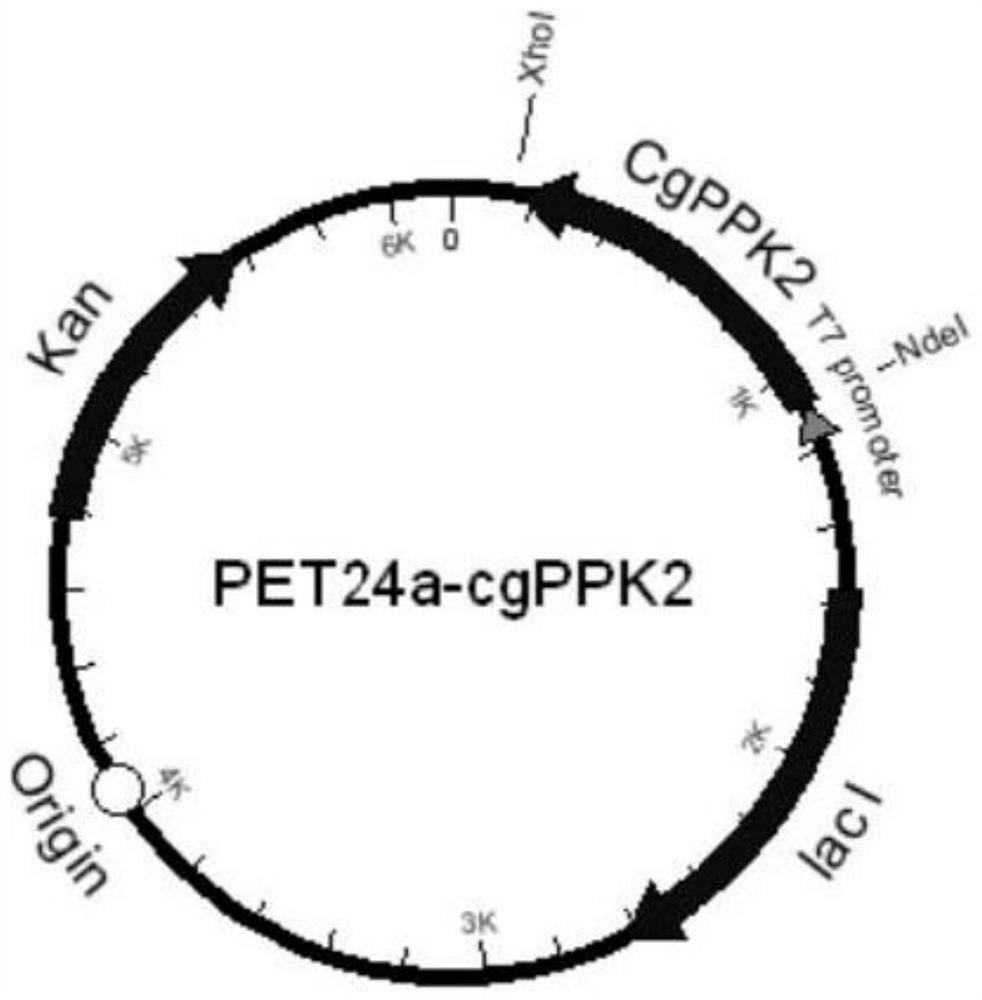
[0055] For the wild-type polyphosphate kinase (GenBank sequence number is NP_601909) derived from Corynebacterium glutamicum ATCC 13032, namely SEQ ID NO: 3, codon optimization is carried out on this basis, and the whole gene synthesis gene sequence SEQ ID NO: 4. Design restriction endonuclease sites Nde I and XhoI at both ends of the gene, subclone into the corresponding sites of the vector pET24a (Novagen), and obtain the recombinant plasmid pET24a-cgPPK2, see the plasmid map figure 1. The recombinant plasmid pET24a-cgPPK2 was transformed into the expression host Escherichia coli BL21(DE3) to obtain the recombinant Escherichia coli PET24a-cgPPK2 / BL21(DE3) expressing wild-type polyphosphate kinase.
Embodiment 2
[0056] Example 2 Construction of Escherichia coli high-throughput screening host
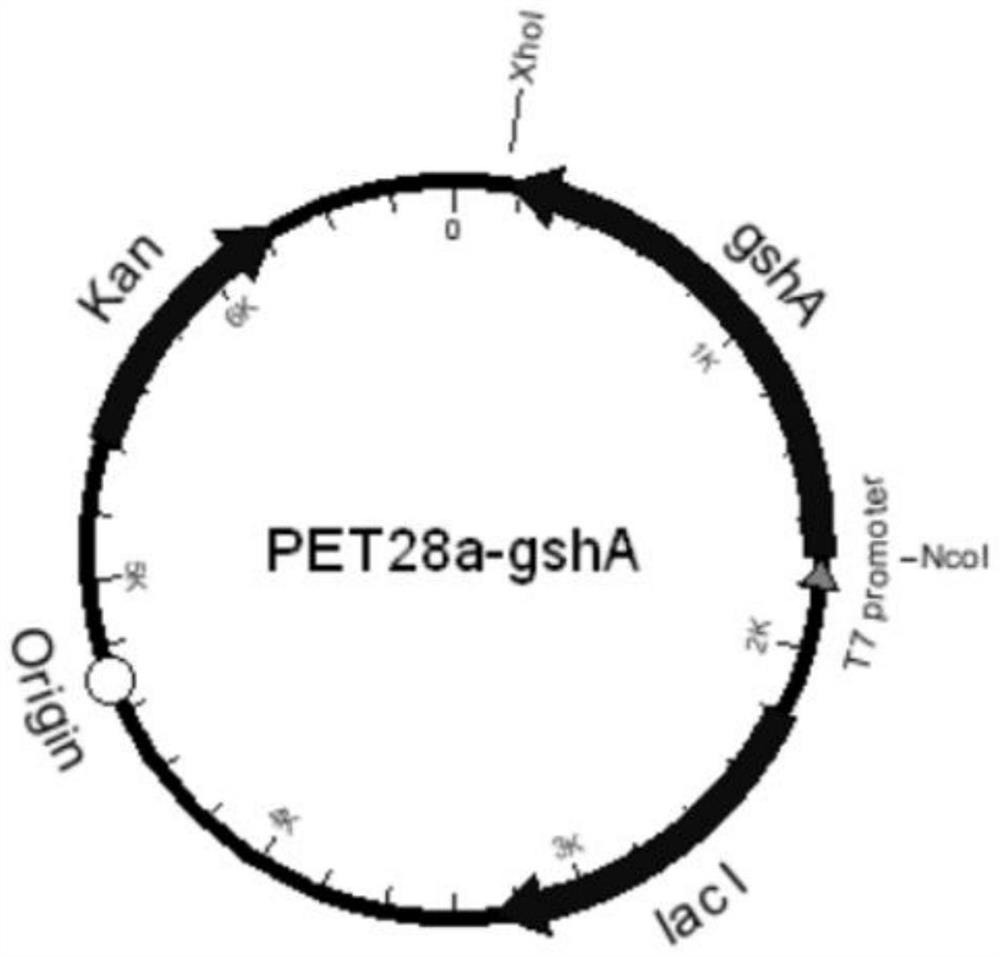
[0057] The gshB in BL21(DE3) was knocked out by insertion and knockout by Red homologous recombination, and the combined fragments of glutamic acid-cysteine ligase gene gshA and chloramphenicol gene were inserted into glutathione Peptide synthase gene gshB site.
[0058] According to the gene sequence (NCBI accession number: AM946981.2) of the glutamate-cysteine ligase gene gshA derived from Escherichia coli BL21 (DE3), the primers were designed as follows:
[0059] Forward GshA-NcoI-F: 5'-CATGCCATGGGAATCCCGGACGTATCACAGGC-3',
[0060] Reverse GshA-Xho I-R: 5'-CGCTCGAGTCAGGCGTGTTTTTCCAGCC-3'.
[0061] The gshA fragment was amplified using BL21(DE3) genomic DNA as a template.
[0062] The PCR reaction system includes: 50pmol each primer, 100ng BL21(DE3) DNA template, 1×KOD neo plusbuffer, 0.2mM dNTP, 1.5mM MgSO 4 , KOD neo plus 1U, add ddH 2 O to 50 μL of the total system. The PCR amplif...
Embodiment 3
[0083] Embodiment 3 error-prone PCR method constructs random mutation library and screening method
[0084] 3.1 The first round of error-prone PCR method to construct random mutation library
[0085] Using the gene SEQ ID NO:4 of the wild enzyme as a template, an error-prone PCR technique was used to construct a random mutant library. The forward primer cgPPK2-F is 5'-ATGGTTGGTAAACTGCCGATC-3', and the reverse primer cgPPK2-R is 5'-TTAGTCACCGATCTGGTCACG-3'.
[0086] 50μL error-prone PCR reaction system includes: 10ng plasmid template pET24a-cgPPK2, 50pmol pair of primers cgPPK2-F and cgPPK2-R, 1×Taq buffer, 0.2mM dGTP, 0.2mM dATP, 1mM dCTP, 1mM dTTP, 7mMMgCl 2 , (0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM) MnCl 2 , 2.5 units of Taq enzyme (Fermentas). The PCR reaction conditions were: 95°C for 5 minutes; 30 cycles of 94°C for 30s, 55°C for 30s, 72°C for 2min / kbp; 72°C for 10min. The 1kbp random mutant fragment was recovered from the gel as a large primer, and MegaPrimer PCR was perf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com