Application of benzbromarone as FXR agonist
A technology of benzbromarone and agonist, applied in the field of new drug use, can solve the problems of poor selectivity, limited clinical development, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
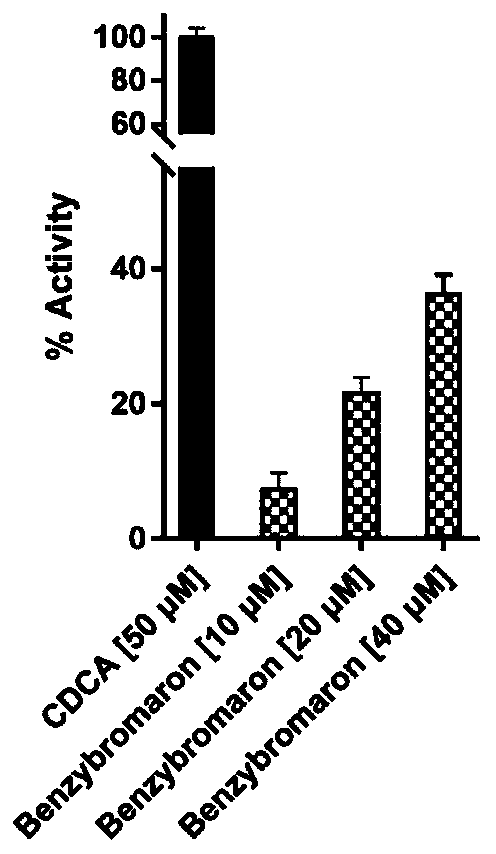
[0016] Example 1: HTRF homogeneous time-resolved fluorescence technique.
[0017] The test system in the 384-well plate contains 10nM GST-FXRαLBD, 100nM biotin-SRC1, 0.83nMEu-labeled anti-GST (Cisbio) and 41.75nM Streptavidin-XL665 (Cisbio), and the test buffer consists of 50mM Hepes pH 7.0, 125mM KF, 0.125 % CHAPS and 0.05% milk powder, adding different concentrations of the test compound, incubating at room temperature for 1 hour, using a multi-functional microplate reader to detect the fluorescence value at 620nm and 665nm, the measured value is represented by (665nm / 620nm*10000), Three replicate wells were set up for each compound, 50 μM CDCA was used as the positive control drug, DMSO was used as the blank control, and the experimental results of homogeneous time-resolved fluorescence (HTRF) were shown in figure 1 .
Embodiment 2
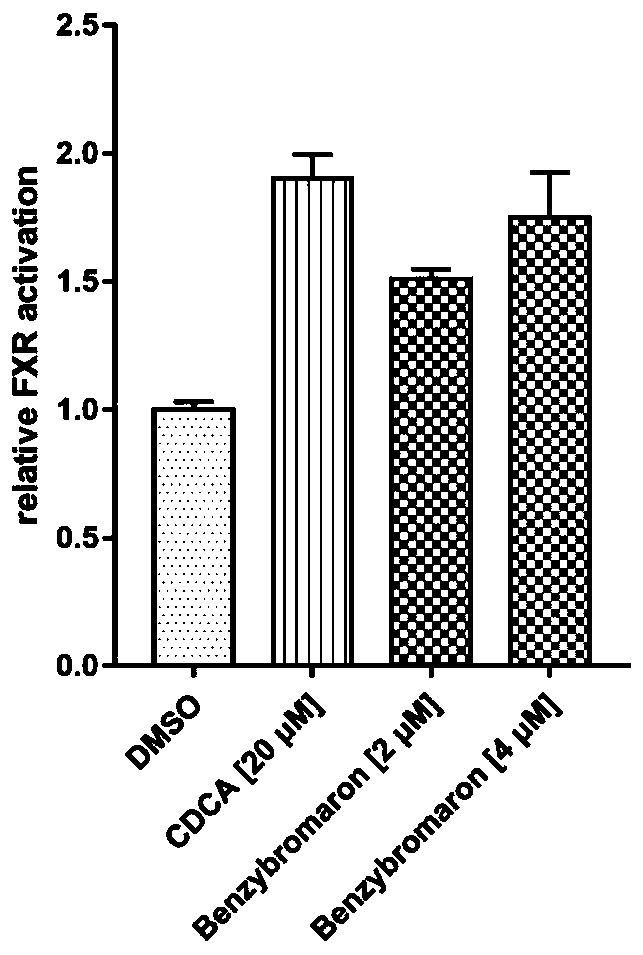
[0018] Example 2: Transcription activation assay.
[0019] HEK293T cells were seeded in 24-well plates, and when they grew overnight to a density of 50%-60%, the medium was replaced with serum-free DMEM medium. Firstly, the reporter gene plasmids pCDNA3.1-FXR (400ng / well), pCDNA3.1-RXRα (400ng / well), pGL3-FXRE-luc (400ng / well) and PRL-SV40 (100ng / well) were mixed with CaCl 2 (20 μL / well) and mix well, then add the mixture to BBS (20 μL / well) and mix well for 15-30 minutes, and finally 40 μL / well are co-transfected into the cells. After 6 hours, the medium was changed, and 20 μM CDCA or 2 μM benzbromarone were given for 24 hours at the same time. After 24 hours, the medium was removed, the cells were gently washed twice with PBS, and then the cells were lysed with diluted lysis buffer for 15-20 minutes, and the luciferase activity was measured with Dual Luciferase Assay System Kit (Promega). Such as figure 2 The results showed that the agonistic activity of benzbromarone on...
Embodiment 3
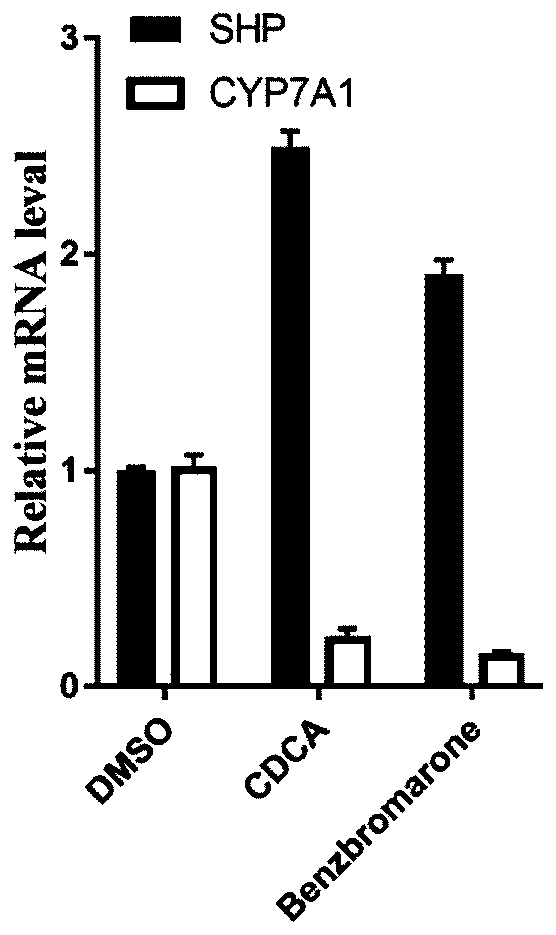
[0020] Example 3: qRT-PCR experiment detects the expression level of FXR-related genes.
[0021] HepG2 cells were incubated for 8 h in the presence of the test compound or control drug, the cells were collected, washed with PBS buffer, and then used for RNA extraction. RNA was extracted from HepG2 cells using a total RNA extraction kit (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA), and then reverse-transcribed into cDNA according to the instructions of the reverse transcription kit (Applied Biosystems, FosterCity, CA). Finally, the FXR-related genes were analyzed by real-time quantitative PCR instrument. The results show image 3 , benzbromarone can significantly increase the expression of SHP and reduce the content of CYP7A1, which is consistent with the results of the positive control drug CDCA. The primer sequences used in the quantitative PCR experiment are shown in Table 1.
[0022] Table 1. Primer sequences used in quantitative PCR experiments.
[0023]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com