Environment-friendly preparation method of ozagrel impurity II
An environmentally friendly, impurity technology, applied in the field of pharmaceutical impurity standard preparation, to achieve the effects of improving accuracy and sensitivity, convenient operation, and wide linear range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] An environment-friendly preparation method of ozagrel impurity II, comprising the following synthetic route:
[0031]
[0032] The steps are as follows:
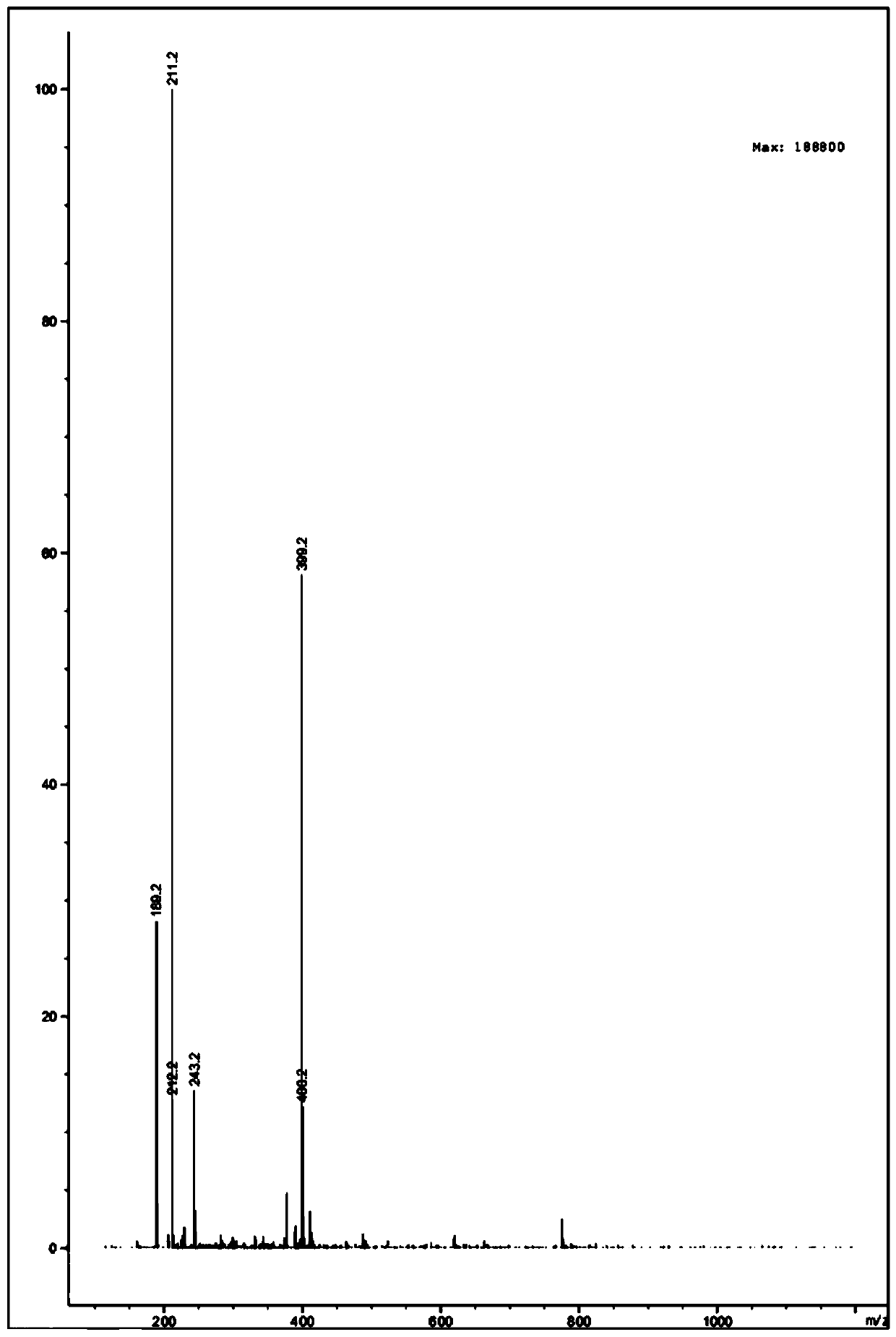
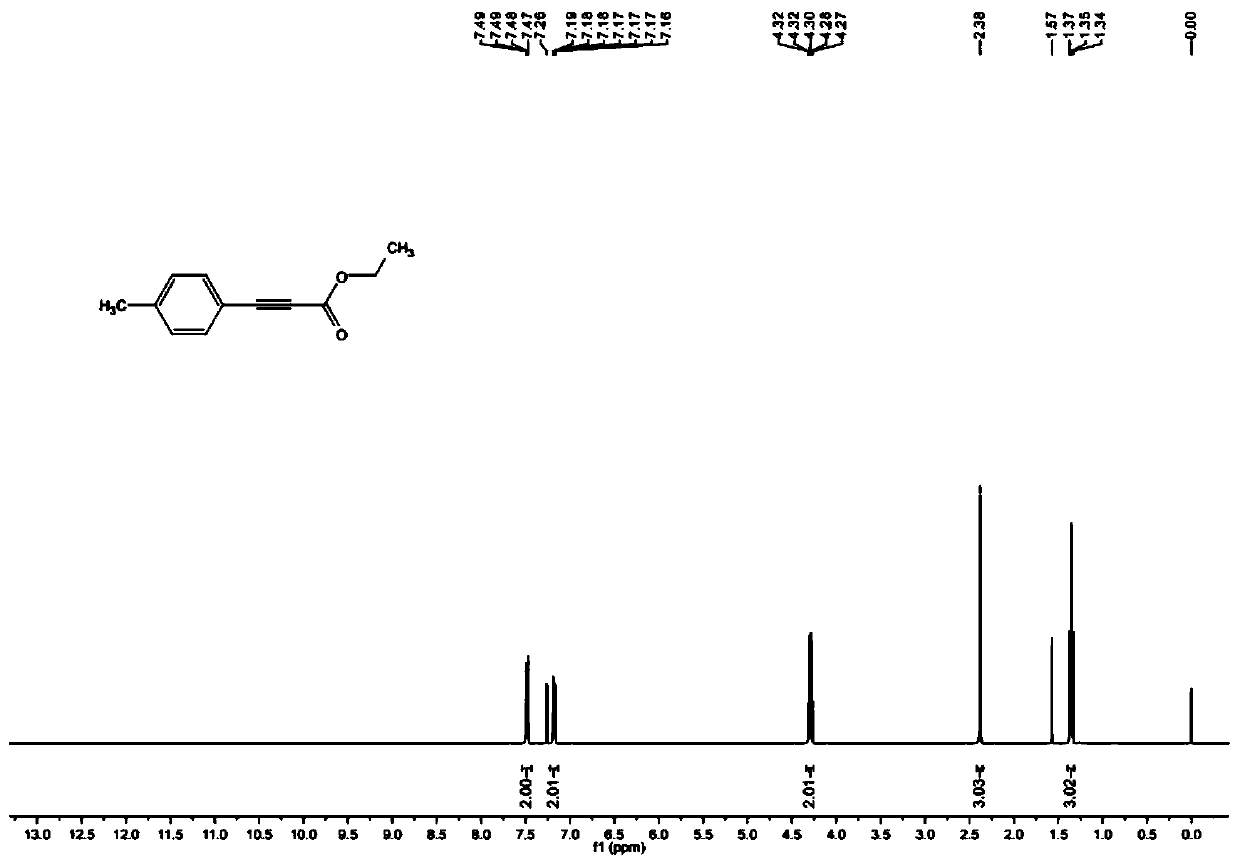
[0033] 4-iodotoluene and ethyl propiolate on Cu 2 Ozagrel impurity II was obtained by the reaction under the catalysis of O.
[0034] The preparation method of the invention has simple steps, cheap and easy-to-obtain raw materials, mild reaction conditions, stable process, good reproducibility, emission reduction and environmental protection, high product yield, high purity of the obtained ozagrel impurity II, good stability performance, and can be used as The standard is used to identify the impurity Ⅱ produced in the synthesis of ozagrel, effectively detect the residual amount of the impurity Ⅱ of ozagrel in the synthesis product of ozagrel, which is a necessity for the quality control of ozagrel, and to carry out qualitative and quantitative analysis of related substances detection and control.
[0035] In so...
Embodiment 1
[0053] An environment-friendly preparation method of ozagrel impurity II, specifically comprising the following steps:
[0054] (1) Add 25 mL of dry dimethylformamide to 2.00 mL of ethyl propiolate, then add 2.18 g of 4-iodotoluene and mix well, then add Cu 2 O 2.86g, the system was subjected to vacuum / nitrogen circulation degassing operation 3 times, and then the system was stirred and reacted at 100°C for 16h in a nitrogen protection environment;
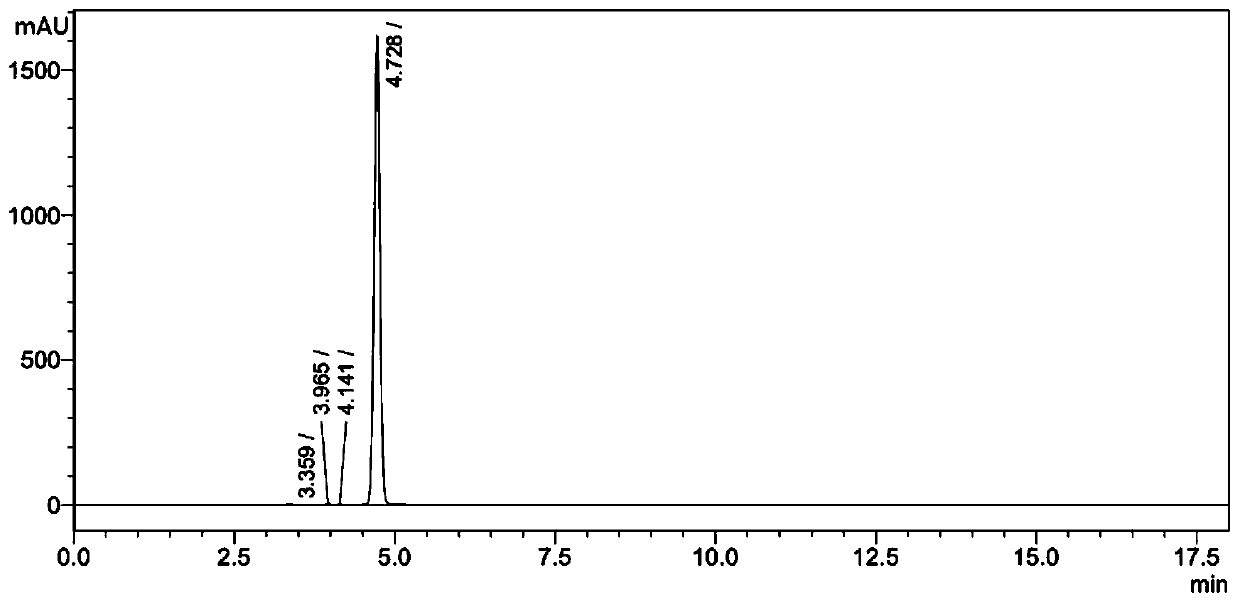
[0055] (2) The mixture system that completes the reaction is cooled to room temperature, then vacuumizes to remove solvent, then adds 30ml saturated NaCl solution to wash, and adopts 50ml dichloromethane to extract, gained organic matter is washed with NaCl 2 SO 4 Dry, filter, and concentrate in vacuo to obtain a crude product, which is purified by thin-layer chromatography. The developer ratio for chromatography is petroleum ether:ethyl acetate=10:1, and 760.00 mg of a yellow liquid product-Ozagrel impurity II is obtained. The ...
Embodiment 2
[0057] An environment-friendly preparation method of ozagrel impurity II, which differs from Example 1 only in that:
[0058] In step (1): Cu 2 The amount of O added is 3.00g, and the system is subjected to vacuum / nitrogen cycle degassing operation twice, and then the system is stirred and reacted at 105°C for 15h in a nitrogen protection environment;
[0059] In step (2): the crude product is purified by thin-layer chromatography, and the ratio of developing solvent for chromatography is petroleum ether:ethyl acetate=12:1, and 769.41mg of yellow liquid product-Ozagrel impurity II is obtained, and the yield of the product is was 40.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com