Bifunctional oxidation electrocatalyst and preparation method thereof
An electro-oxidation and dual-function technology, applied in the field of electrochemistry, can solve problems such as inability to fully exert the metal activity, and achieve the effect of ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 CoNiO x Synthesis of @C / G-NSs
[0033] (1) Take a clean beaker, pour 75mL of methanol; wash and dry the medicine spoon with deionized water and ethanol, take 7.96g of cobalt nitrate hexahydrate 0.027 with an electronic balance, add it into the beaker, stir to dissolve, and weigh with an electronic balance 3.88g nickel nitrate hexahydrate was added into a beaker for dissolution to obtain solution I. Take another clean beaker and pour 75mL of methanol into it; wash and dry the spatula with deionized water and ethanol, take 6.16g of dimethylimidazole with an electronic balance, add it into the beaker and stir to dissolve to obtain solution II. Then, solution II was slowly added to solution I under stirring to obtain gray-purple mixed solution III;
[0034] (2) Put solution III in an oven and dry it at 80°C for 48 hours to obtain gray-purple precursor crystals;
[0035] (3) Take three 2g precursor crystals, put them into a porcelain boat, and then put them into...
Embodiment 2
[0036] Example 2 CoNiO x Composition and structure of @C / G-NSs
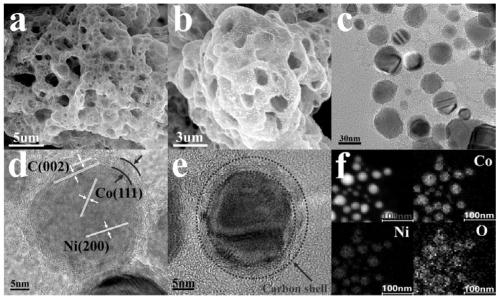
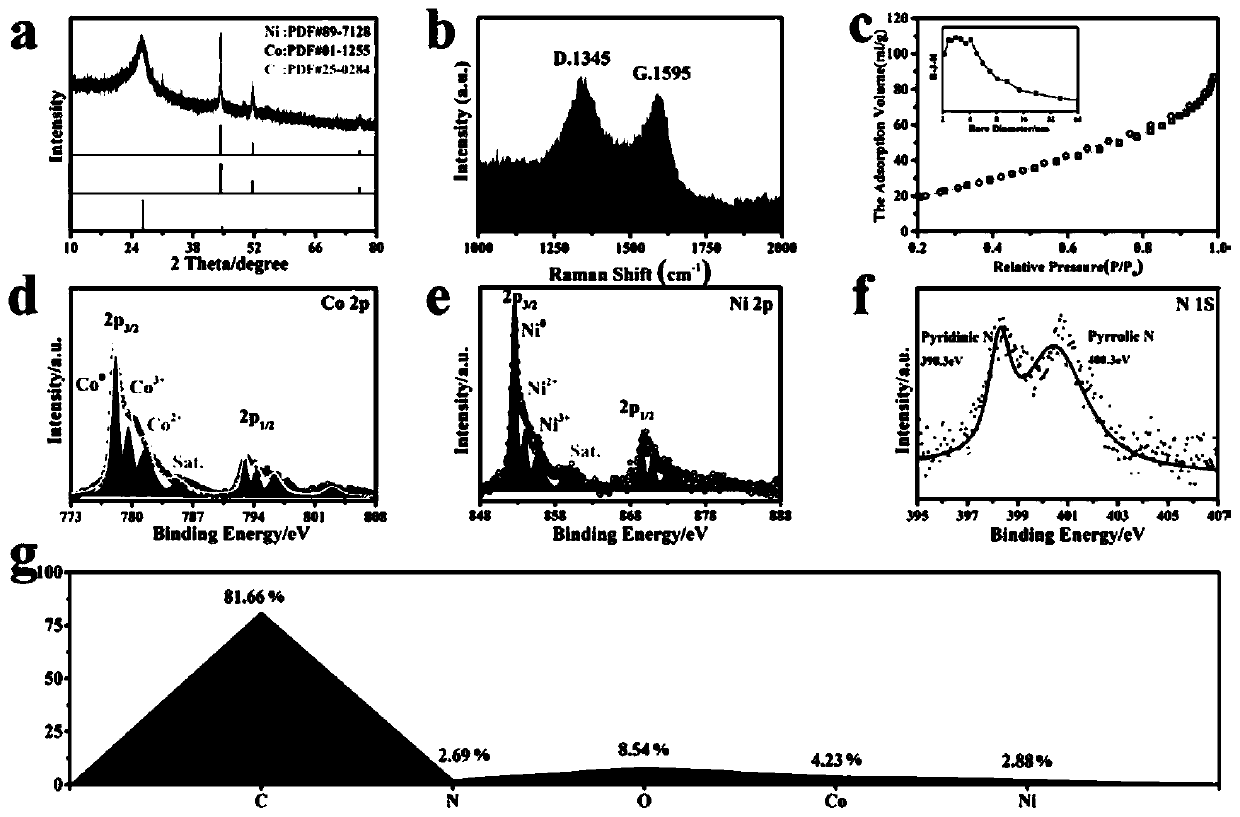
[0037] figure 1 a-b are CoNiO photographed by scanning electron microscope x Microscopic topography of @C / G-NSs, showing CoNiO x @C / G-NSs graphene-like three-dimensional fluffy and hierarchical porous nanostructure provides a high-speed channel for mass and electron transport in the process of oxygen reduction reaction and oxygen evolution reaction, with higher conductivity and better material transport ability. figure 1 c-d are CoNiO taken by transmission electron microscope x @C / G - NSs diagram, from figure 1 b-c It can be clearly seen that a large number of cobalt nickel oxide nanoparticles with a diameter of 30-50nm are distributed on the graphene-like nanoflakes, and smaller and more uniform nanoparticles are beneficial to improve the catalytic activity. figure 1 d-e show that the thickness of cobalt-nickel oxide nanoparticles coated with carbon shells is about 4 nm, and in CoNiO x In @C / G-NSs, nanopa...
Embodiment 3
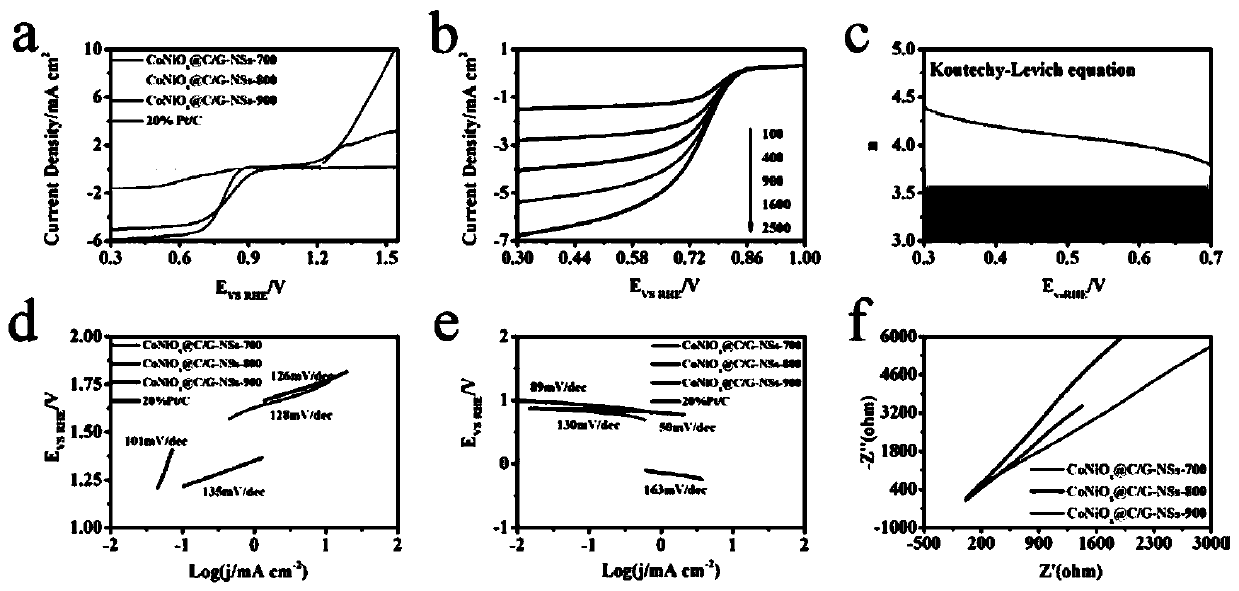
[0039] Example 3 CoNiO at different pyrolysis temperatures x Oxygen evolution reaction and oxygen reduction reaction catalytic performance of @C / G-NSs
[0040] The test method is as follows: all electrochemical data are tested using CHI760E electrochemical workstation; at room temperature, three electrodes are tested in 0.1 M KOH solution; the working electrode is a rotating ring disk electrode, and the reference electrode is saturated Hg(l) | Hg 2 Cl 2 (saturated KCl solution) electrode; the counter electrode is a platinum wire.
[0041] Dissolve 5 mg of the catalyst in 1 mL of absolute ethanol, and drop 10 uL of the catalyst solution on the surface of the working electrode for electrochemical testing.
[0042] All potentials involved in this experiment are referenced to the reversible hydrogen electrode (RHE), calculated as E(RHE) = E(Hg(l)|Hg 2 Cl 2 , saturated KCl solution) + pH*0.059V + 0.241V. Hg(l) | Hg compared to RHE 2 Cl 2 (saturated KCl solution) The referen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com