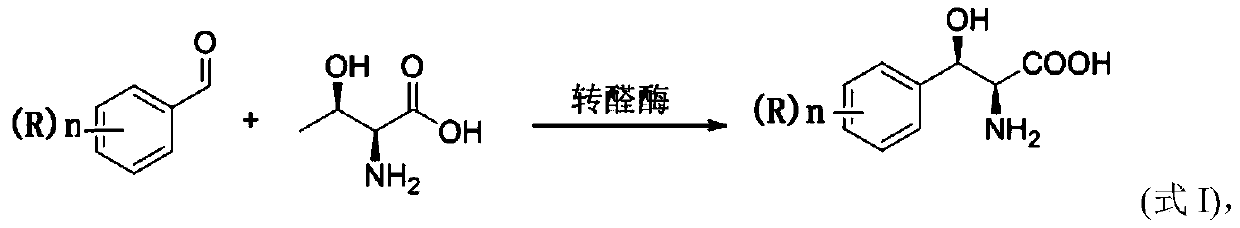
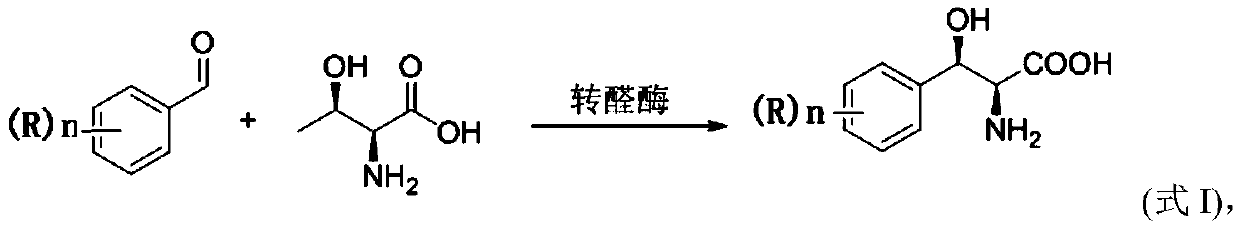
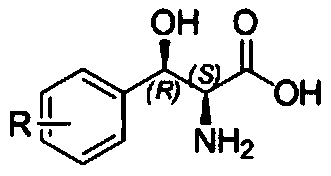
Method for preparing 3-phenyl-L-serine or derivative thereof and ethyl ester of 3-phenyl-L-serine
A technology for serine and its derivatives, which is applied in the field of preparation of chiral 3-phenyl-L-serine or its derivatives and its ethyl ester, which can solve problems such as low conversion rate, lack of industrialization conditions, poor stereoselectivity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Gene cloning and expression vector construction
[0112] Four wild-type L-threonine transaldolases, namely: YH1058 (Suzhou Pivot Biotechnology Co., Ltd., amino acid sequence shown in SEQ ID NO.12), and LTTA sp01 (amino acid sequence shown in SEQ ID NO.13 shown), LTTA sp02 (amino acid sequence as shown in SEQ ID NO.14) and LTTA sp03 (amino acid sequence as shown in SEQ ID NO.15) gene sequences can be obtained in the NCBI database, by means of gene synthesis and molecular cloning Construct pET30a expression plasmid containing threonine transaldolase gene. The recombinant plasmid was transformed into Escherichia coli BL21 (DE3) cells to obtain recombinant bacteria.
Embodiment 2
[0113] Embodiment 2: Expression of recombinant L-threonine transaldolase
[0114] Inoculate the recombinant bacteria in Example 1 into LB medium (10 g / L peptone, 5 g / L yeast powder, 10 g / L NaCl, pH 7.0) containing 50 μg / mL kanamycin, and culture overnight at 37°C. The overnight culture was transferred to TB medium (peptone 12g / L, yeast extract 24g / L, glycerol 4mL / L, potassium dihydrogen phosphate 2.31g / L, dipotassium hydrogen phosphate 12.54g / L), and cultured at 37°C until OD 600 To 0.6-0.8, add IPTG with a final concentration of 0.4mM, and induce expression overnight at 30°C. Cells were collected by centrifugation and resuspended in 20mM pH 7.0 phosphate buffer 4 times the wet weight of the cells, sonicated to break the cells, centrifuged, and the supernatant was taken for reaction or frozen at -20°C.
Embodiment 3
[0115] Example 3: Test of wild-type L-threonine transaldolase activity at low substrate concentration - no acetaldehyde removal system
[0116] In the 1mL reaction system, add the final concentration of 5g / L p-thiamphenicol benzaldehyde, 6.4g / L L-threonine, 1g / L magnesium chloride, 0.005g / L pyridoxal phosphate, 100mM phosphate buffer (pH7. 5) Heat to 30°C and stir evenly with magnetic force, add 20 μl of the L-threonine transaldolase obtained in Example 2 (YH1058 (Suzhou Pivot Biotechnology Co., Ltd.), LTTA sp01, LTTA sp02 and LTTA sp03) enzyme solution, start stirring reaction, after 16 hours, sampling HPLC detects, and test result is shown in Table 5.
[0117] Table 5 Wild-type L-threonine transaldolase activity detection results
[0118] Numbering serial number Conversion rate(%) Enantiomeric excess (ee%) a
Diastereomeric Ratio (DR) b
YH1058 SEQ ID NO.12 93.6 98.6 94.7:5.3 LTTA sp01 SEQ ID NO.13 91.5 98.5 94.2:5.8 LTTA sp0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com