Continuous preparation method of high-selectivity alpha-ionone
A technology of pseudo-ionone and ionone, applied in the field of fragrance chemistry and fine chemicals, can solve the problems of low production efficiency, high price of organic acid, high production cost, etc., and achieve the goal of improving production efficiency, high catalytic efficiency and cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 A continuous preparation method of highly selective α-ionone
[0044] Include the following steps:
[0045] The catalysts used include concentrated sulfuric acid and isovaleric acid, which are respectively marked as catalyst 1 and catalyst 2; the catalyst auxiliary agent used is benzyltriethylammonium chloride.
[0046] Weigh 36 kg of isovaleric acid and 3.6 kg of benzyltriethylammonium chloride, add them into a stirring tank and stir evenly; then slowly add 36 kg of concentrated sulfuric acid with a mass percentage of 85% to obtain a catalyst system.
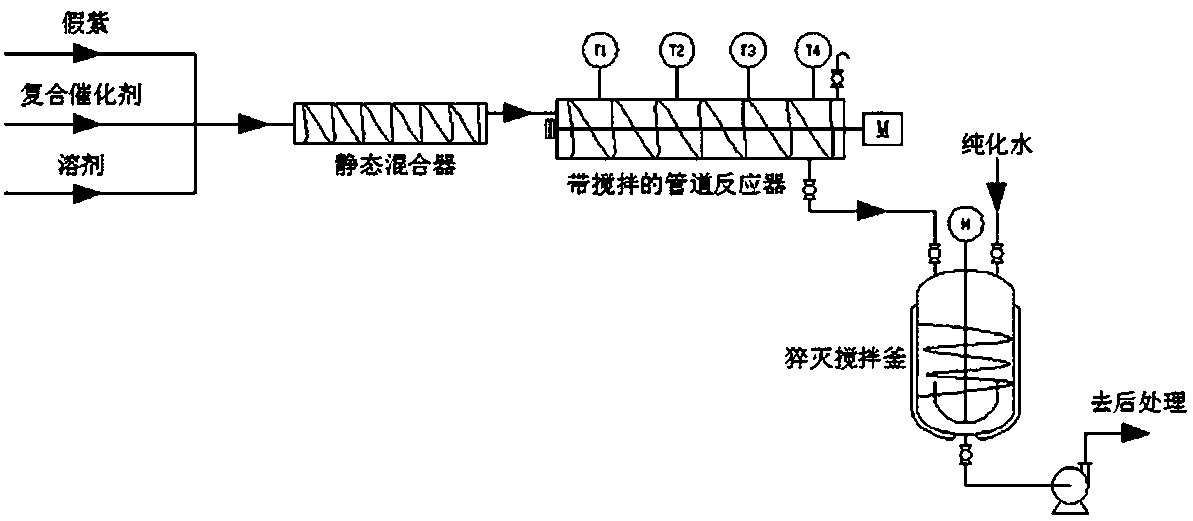
[0047] The catalyst system is passed through the low-temperature tank to control the temperature of the kettle at -2°C-2°C (the low-temperature tank and the jacket of the stirred tank are connected by pipelines to form a loop), and the stirring is continued to prevent local overheating.
[0048] The solvent used was toluene.
[0049] Weigh 72 kg of industrial-grade toluene and 36 kg of pseudoionone, and add t...
Embodiment 2
[0060] Example 2 A continuous preparation method of highly selective α-ionone
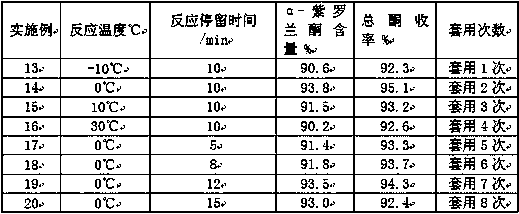
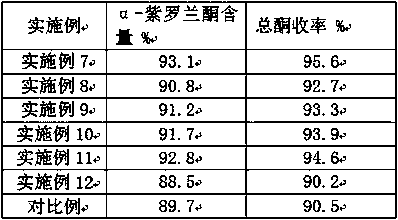
[0061] Same as the preparation method of Example 1, only change the catalyst, auxiliary agent, solvent, material proportion and reaction conditions, carry out the preparation method of embodiment 2-6; The catalyst, auxiliary agent, solvent, material used in embodiment 1-6 The proportioning ratio is shown in Table 1, and the reaction conditions used in Examples 1-6 are specifically shown in Table 2; the ionone finished product obtained after the reaction of Examples 1-6 is detected by gas chromatography, and the α-ionone of the reaction product is obtained. content, post-processing to obtain the total ketone yield, specifically as shown in Table 3;
[0062] Table 1 Embodiment 1-6 reaction material composition and material proportion
[0063]
[0064] The reaction condition of table 2 embodiment 1-6
[0065]
[0066] Table 3 The α-ionone content and total ketone yield of the reaction product ...
Embodiment 7
[0069] Example 7 A continuous preparation method of highly selective α-ionone
[0070] (1) Preparing the catalyst system
[0071] The catalysts used include methanesulfonic acid and isovaleric acid, which are respectively marked as catalyst 1 and catalyst 2; the catalyst auxiliary agent used is benzyltriethylammonium chloride; the solvent used is toluene. The mass percent content of the methanesulfonic acid is 99%.
[0072] Weigh 36 kg of isovaleric acid and 3.6 kg of benzyltriethylammonium chloride, add them into a stirring tank, and stir for 5 minutes at a stirring rate of 200 r / min. Then slowly add 36 kg of methanesulfonic acid with a mass percent content of 99% to obtain a catalyst system.
[0073] (2) Reaction material pretreatment
[0074] The catalyst system is passed through the low-temperature tank to control the temperature of the kettle at -2°C-2°C (the low-temperature tank and the jacket of the stirred tank are connected by pipelines to form a loop), and the sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com