Peptide calcium chelate and preparation method and application thereof
A chelate and chelation reaction technology, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of low development and utilization of bonito flakes protein.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1, Preparation and Characteristic Analysis of Bonito Protein Peptide Powder
[0056] 1. The preparation method of bonito protein peptide powder
[0057] The present embodiment provides a preferred method for preparing Auxis thazard, and the specific operations are as follows. The meat of the flat rudder skipjack is taken, minced, homogenized, and then enzymatically hydrolyzed by papain and flavor protease. The enzymatic hydrolysis conditions are: temperature 50°C, papain addition amount 500U / g, flavor protease addition amount 200U / g, pH 6.5 , Enzymolysis time 3h. The enzymolyzed product was centrifuged for 15min (3500g, 4°C) after the enzyme was inactivated in a water bath, and the obtained supernatant was deoiled by a tubular centrifuge, decolorized by perlite (addition amount was 3%), and then passed through a ceramic membrane (pore size 0.2μm) and ultra Filtrate with a filter membrane (5000U), and finally obtain the bonito oligopeptide powder through concen...
Embodiment 2
[0070] Example 2, preparation and structural characterization of bonito protein peptide chelated calcium
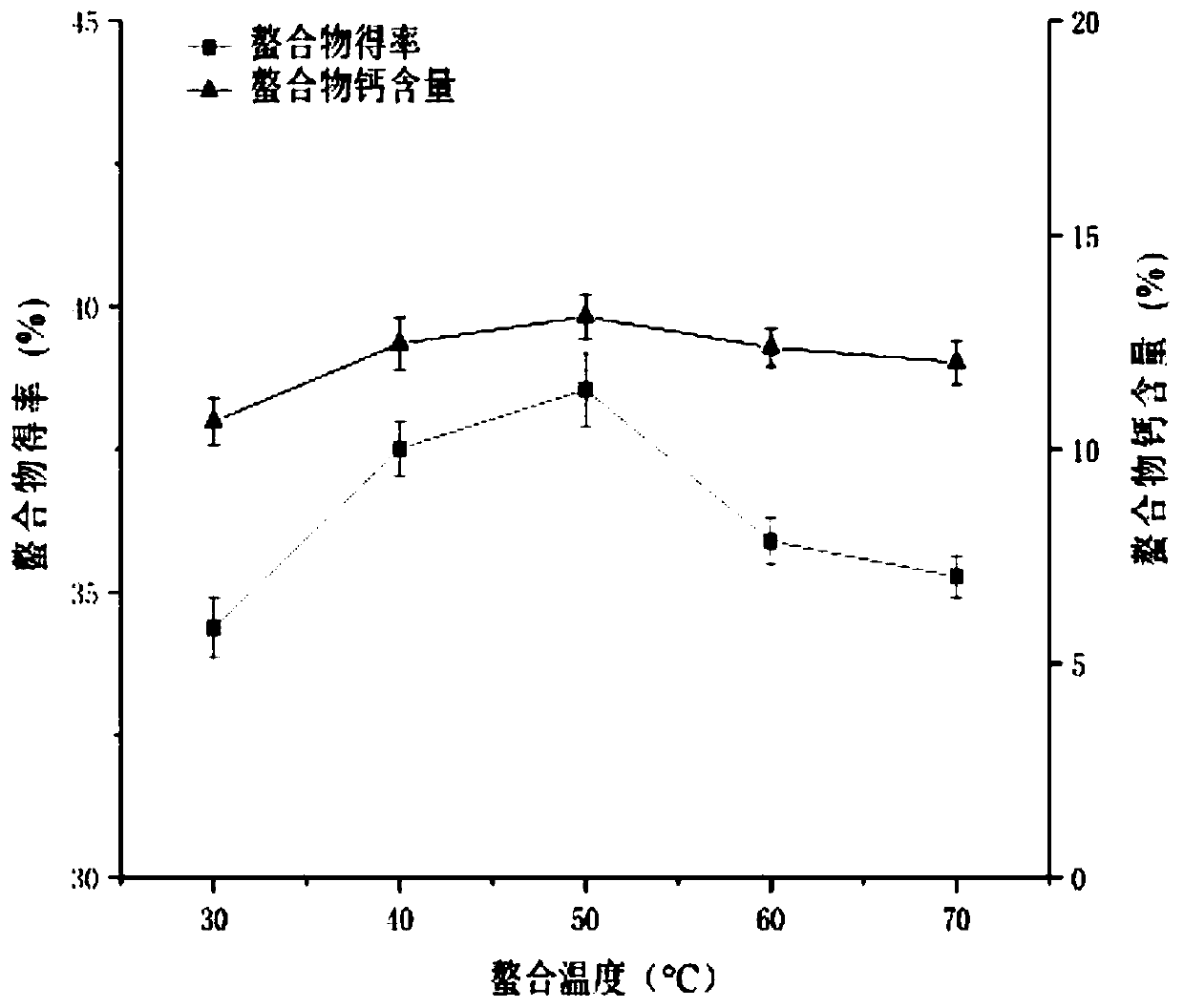
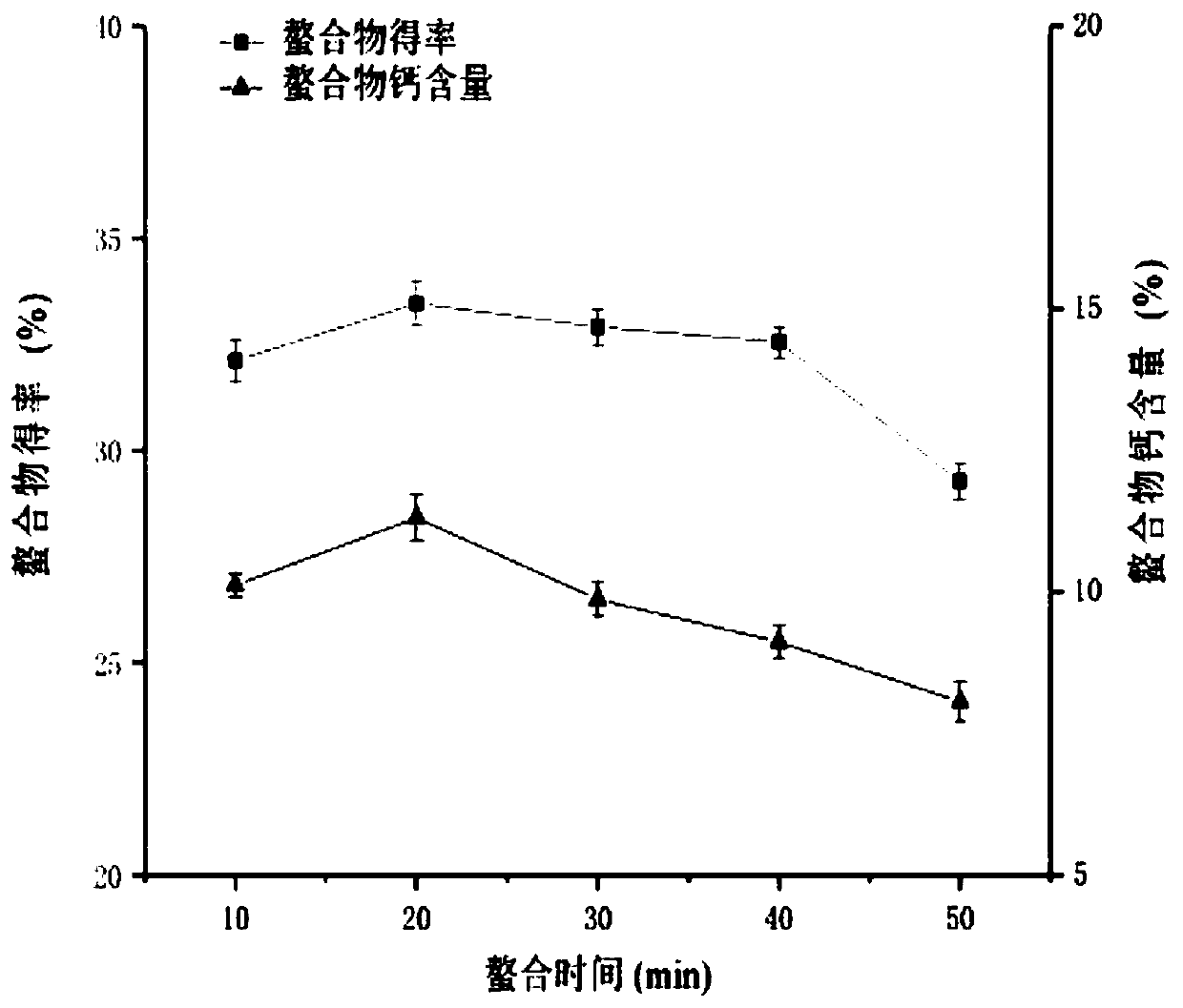
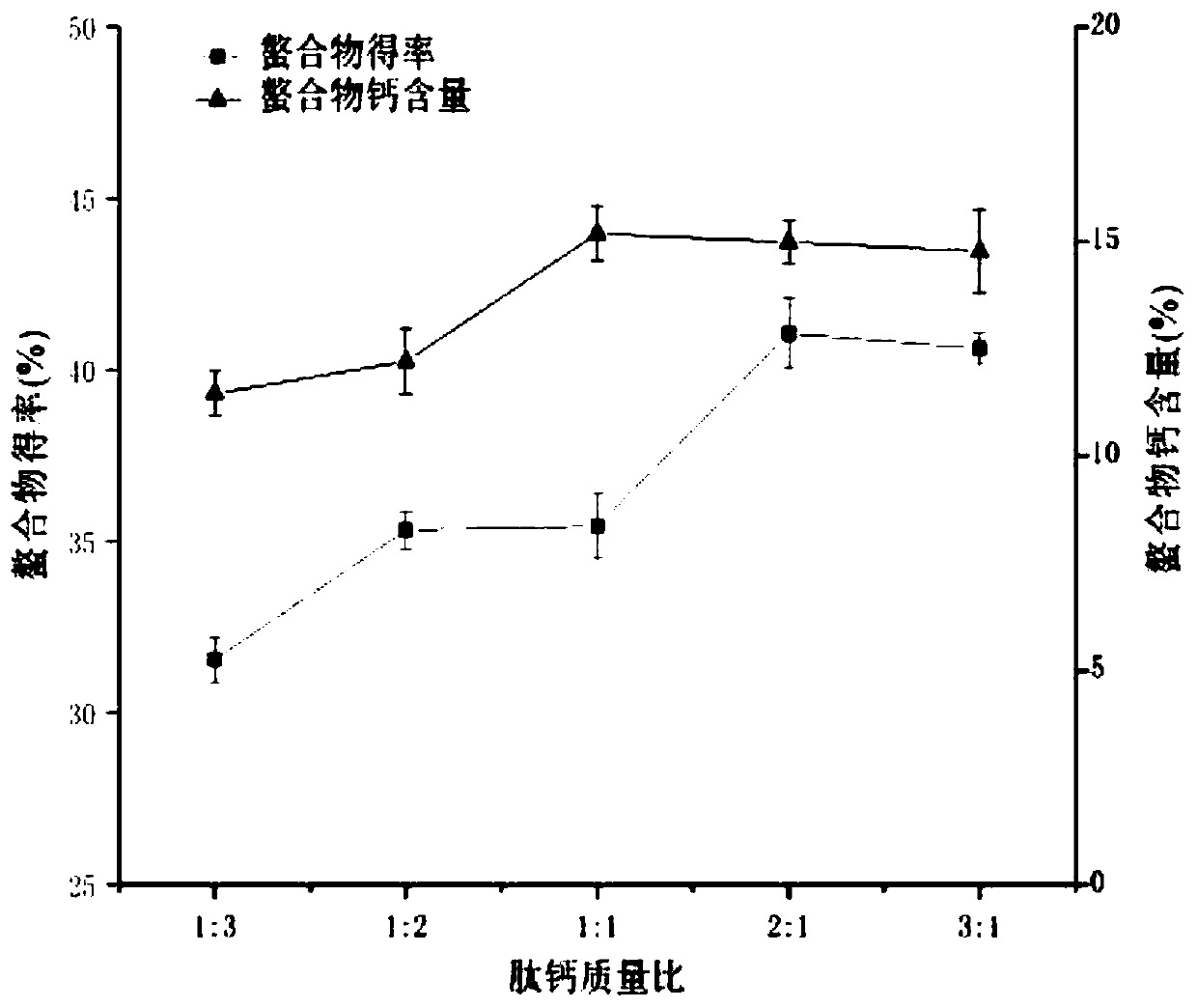
[0071] The preparation yield of protein peptide chelated calcium is closely related to the amino acid composition and molecular weight of the peptide source, as well as the type of calcium source and preparation conditions. The bonito protein peptide chelated calcium prepared in this embodiment selects the bonito protein peptide described in Example 1 as a protein source, and there are many types of calcium sources, among which calcium chloride and hydroxide are the most widely used. Calcium, calcium chloride is selected as the calcium source of the present embodiment. It should be noted that the type of calcium source is not particularly limited in this embodiment. Based on the technical ideas of the present invention, those skilled in the art can prepare the calcium source described in this embodiment by selecting one or more specific calcium sources. Skipjack protein ...
Embodiment 3
[0089] Embodiment three, separation and purification of calcium binding peptide
[0090] In this example, Sephadex G-15 gel chromatography and RP-HPLC were used to separate and purify protein peptides with high calcium binding activity from bonito protein peptides, and to study its binding characteristics with calcium ions by infrared spectroscopy and mass spectrometry .
[0091] Skipjack oligopeptides were separated by Sephadex G-15 gel ( Figure 12 ), to obtain 5 different fractions, respectively denoted as F1, F2, F3, F4, F5, the calcium binding activity of which is as follows Figure 13 shown. As the molecular weight decreases, the calcium-binding ability of the peptide gradually increases. The first four components have strong calcium-binding activity, and component F4 shows the strongest calcium-binding ability (73.6±4.6mg / g). The calcium-binding activity of F5 was significantly lower than that of the other four components. It was speculated that the main components o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com