Preparation and application of novel amphoteric chiral selector CEC monolithic column
A technology of chiral selector and monolithic column, which is applied in the field of preparation and application of bis[-6-N-]-β-CD chiral CEC column, can solve the problem of few research reports on the separation of chiral pesticides by CEC, and achieve Low cost and the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
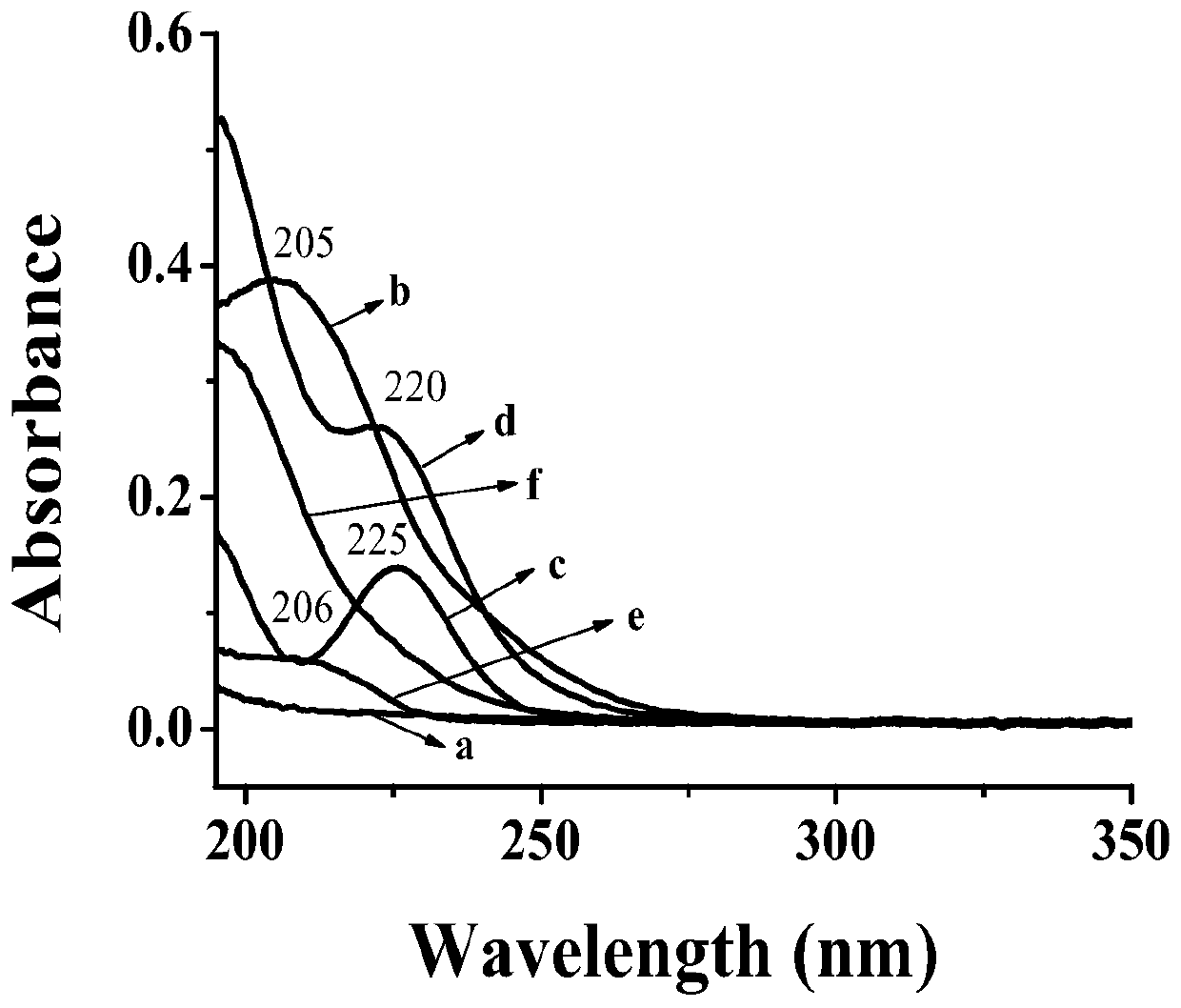
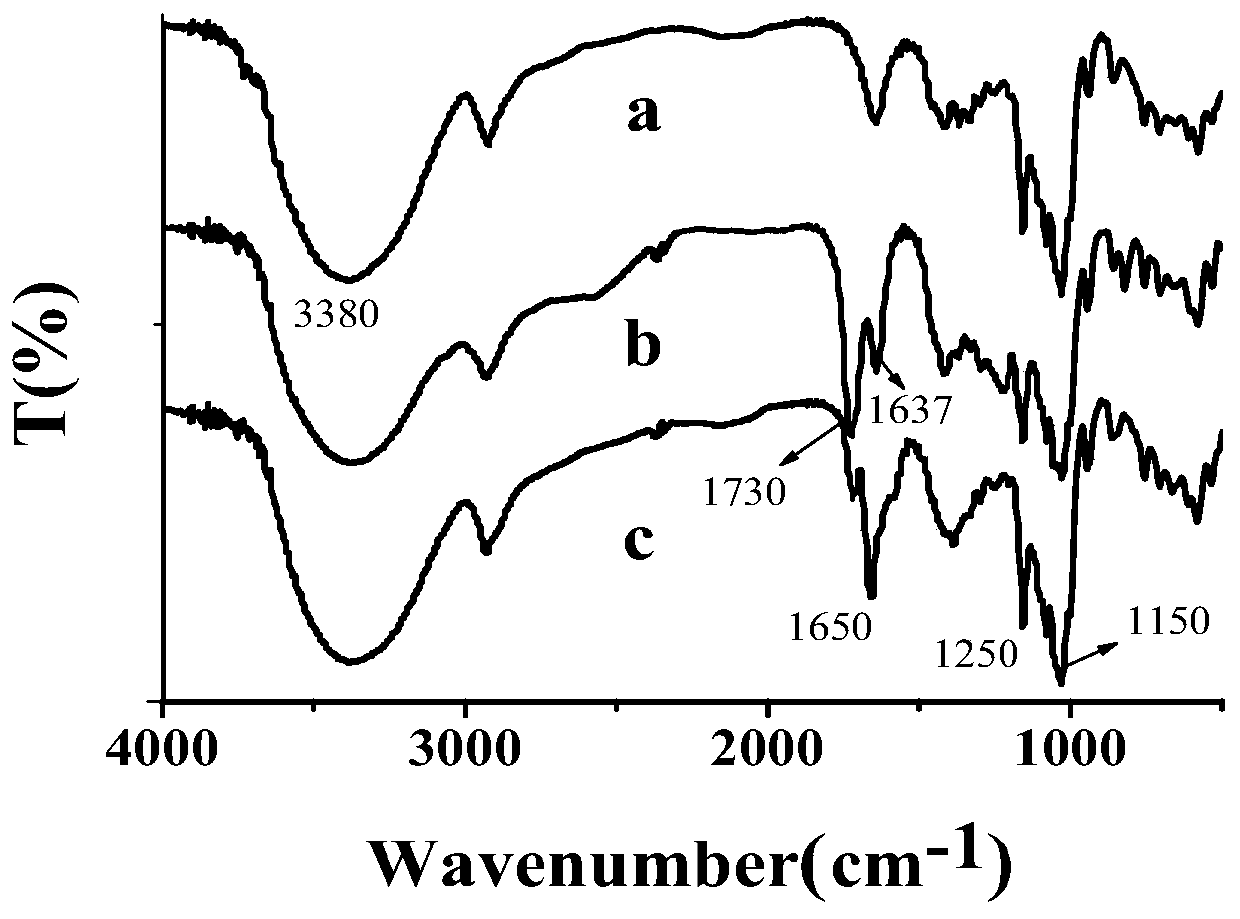
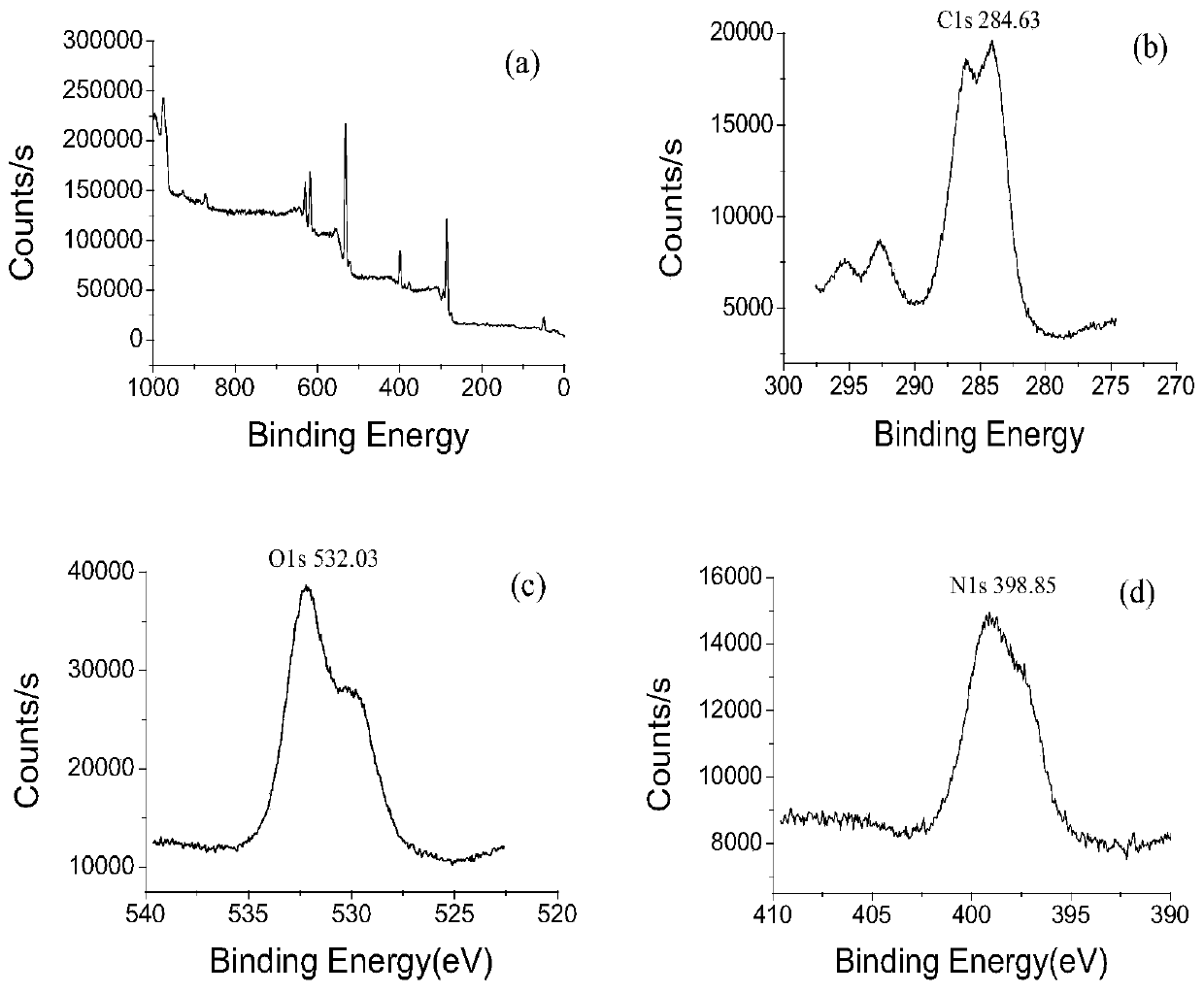
[0045] Example 1: Preparation and characterization of bis[-6-nitrogen-(imidazolyl-2-amino-propionic acid-3-)]-β-CD
[0046] According to the literature (Song Fajun, Ding Zhigang et al. Metal complexes of β-cyclodextrin derivatives catalyze the hydrolysis of RNA. Molecular Catalysis, 2001,15(02):139-142.) Prepare β-CD-E by a three-step method 2 Afterwards, β-CD-E was analyzed by means of ultraviolet spectrophotometry (UV), Fourier transform infrared spectroscopy (FTIR), elemental analysis, nuclear magnetic resonance (NMR), and liquid-mass spectrometry (LC-MS). 2 The monomer was characterized and identified as the desired target compound.
[0047] Specific method: first react maleic anhydride and β-CD (molar ratio 15:1), post-treatment, and dry the product β-CD-A 2 , standby; in order to introduce active substituent groups on the side chain of β-CD, the β-CD-A synthesized according to the above literature 2 The product β-CD-I after reaction with KI 2 Post-processing purificat...
Embodiment 2
[0072] Example 2 Preparation and characterization of bis[-6-nitrogen-(imidazolyl-2-amino-propionic acid-3-)]-β-CD electrochromatographic monolithic column
[0073] β-CD-E 2 The preparation route of the monolithic column is as follows Figure 10 As shown, the "one-pot method" is used to prepare the CEC monolithic column according to the following steps:
[0074] (1) Before the preparation of the monolithic column, pass through methanol, NaOH solution (0.01mol / L), HCl solution (0.01mol / L), H 2 O rinses the capillary (inner diameter 75 μm, length 50 cm, Hebei Yongnian Optical Fiber Factory) in sequence. Then methanol and KH560 are mixed at a volume ratio of 1:1 and injected into the capillary column through nitrogen flow; (2) β-CD-E 2 (400 μg), 1,4-butanediol (129 μL), n-propanol (659 μL), ethylene glycol dimethacrylate (73 μL), glycidyl methacrylate (129 μL), and azobisisobutyronitrile (3mg) into a 10mL vial, fill the resulting mixed solution into a pretreated capillary colu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com