Bionic periosteum, periosteum-bone substitute and preparation method
A bone substitute and bionic bone technology, applied in biochemical equipment and methods, microorganisms, tissue regeneration, etc., can solve problems such as nonunion and delayed healing, and achieve bone regeneration promotion, good application prospects, and good osteogenic induction effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Mix the prepolymer and curing agent in the Dow Corning 184 kit at a mass ratio of 10:1, and place it at 60°C for 2 hours to cure to prepare PDMS. Place PDMS in a plasma cleaner to clean it under an oxygen atmosphere. Before PDMS cell culture, UV sterilize the PDMS substrate, and then incubate with 20μg / mL fibronectin for 6-8h;
[0041] (2) Inoculate MC3T3-E1 cells on the incubated PDMS at a density of 0.5×10 4 / cm 2 , when the cells grow to 80% of the surface, add 20 μg / mL ascorbic acid to the medium, and continue to cultivate;
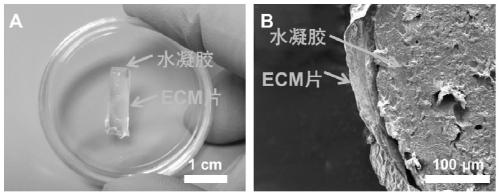
[0042] (3) After 14 days, wash the cultured cell sheet 3 times with PBS, first use 0.25% Triton-X100 and 25mM NH 4 OH solution was used to decellularize the cells for 10min, and then treated with 25U / mL DNase I and 1.5μL / mL RNase A at 37°C for 2h to remove residual cellular DNA and RNA, and the prepared ECM sheets were stored at 4°C for later use. The shape of the sheet is shown in the appendix of the instruction manual. figure 1 ;
...
Embodiment 2
[0046] (1) The prepolymer and curing agent in the Dow Corning 184 kit were mixed at a mass ratio of 20:1, and then cured to obtain PDMS, which was treated with plasma. Before cell culture, the prepared PDMS was sterilized by ultraviolet light, and then incubated with gelatin solution for 8h;
[0047] (2) Inoculate bone marrow mesenchymal stem cells on the hatched PDMS at a density of 2×10 4 / cm 2 , when the cells grow to 90% of the surface, add 50 μg / mL ascorbic acid to the medium to promote the secretion of extracellular matrix, and continue to cultivate until the cell sheet matures;
[0048] (3) Wash the cultured cell sheet 3 times with PBS, first use 0.25% Triton-X100 and 50mM NH 4 OH solution was used to decellularize the cells for 5 minutes, and then treated with 50U / mL DNaseI and 2.5μL / mL RNase A at 37°C for 2h to remove residual cellular DNA and RNA. The ECM sheet has good biocompatibility;
[0049] (4) Add PBS to the GelMA solid sponge, then place it in a 37°C water...
Embodiment 3
[0052] (1) The prepolymer and curing agent in the Dow Corning 184 kit were mixed at a mass ratio of 5:1, and cured to prepare PDMS, which was treated by plasma. Before cell culture, UV sterilize the PDMS substrate, and then incubate with 10μg / mL fibronectin for 6-8h;
[0053] (2) Adipose-derived mesenchymal stem cells were inoculated on the hatched PDMS at a seeding density of 1×10 4 / cm 2 , when the cells grow to 90% of the surface, add 40 μg / mL ascorbic acid to the medium to promote the secretion of extracellular matrix, and continue to cultivate until the cell sheet matures;
[0054] (3) Wash the cultured cell sheet 3 times with PBS, first use 0.1% Triton-X100 and 40mM NH 4 OH solution decellularized the cells for 20 minutes, then treated them with 40 U / mL DNase I and 2.0 μL / mL RNase A at 37 °C for 2 h to remove residual cellular DNA and RNA, and stored the prepared ECM sheets at 4 °C for later use;
[0055] (4) Prepare 2% sodium alginate solution and 10mM calcium chlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com