Sensitive method for determining ethambutol in blood plasma through liquid chromatography-tandem mass spectrometry
A tandem mass spectrometry and ethambutol technology, applied in the field of drug analysis, can solve the problems of time-consuming concentration steps, long analysis time, complicated operation, etc., and achieve the effect of increasing analysis speed, improving safety, and shortening analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Determination of ethambutol in plasma by liquid chromatography-tandem mass spectrometry
[0027] 1. Instrument
[0028] Triple QuadTM 6500+ triple quadrupole tandem mass spectrometer and Analyst 1.6.3 data processing software from Sciex, USA; LC-30 high performance liquid chromatography system from Shimadzu, Japan, including DGU-20A5R degasser, LC-30AD infusion Pump, SIL-30AC autosampler, CTO-20A column thermostat.
[0029] 2. Pretreatment of plasma samples:
[0030] Add 50 µL of ethambutol-d4 internal standard solution (concentration is 100 ng / mL, solvent is methanol:water mixture with a volume ratio of 50:50) to 50 µL plasma sample, and then add 400 µL volume ratio of 90 :10 acetonitrile-water mixture, vortexed for 10 min, centrifuged at 3900 rpm for 10 min at 4°C, and transferred the centrifuged supernatant to another 96-well plate. Take 2.0 µL of the supernatant for LC-MS / MS analysis.
[0031] 3. Preparation of standard series samples and quality c...
Embodiment 2
[0038] Example 2: Methodological Validation
[0039] Carry out methodology verification to the assay method of embodiment 1, specifically as follows:
[0040] 1. Optional:
[0041] The selectivity of the method was evaluated by analyzing the lower limit of quantification samples prepared from 6 different sources of individual blank plasma, hemolyzed plasma, hyperlipidemia plasma and the above blank plasma.
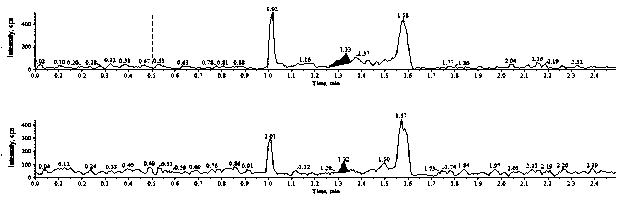
[0042] The results showed that endogenous substances did not interfere with the determination of ethambutol and ethambutol-d4. For a typical chromatogram see image 3 and Figure 4 .
[0043] 2. Standard curve:
[0044] Take the theoretical concentration of ethambutol as the abscissa (x), and the peak area ratio of ethambutol and ethambutol-d4 as the ordinate (y), perform regression analysis to calculate the linear regression equation, weight factor W=1 / x 2 , ethambutol plasma concentration range 4.00 ~ 1600ng . mL −1 Internal linear relationship is good. A typi...
Embodiment 3
[0060] Embodiment 3: clinical sample detection
[0061] The human bioequivalence test of ethambutol hydrochloride tablets was approved by the ethics committee and included 8 healthy subjects. Subjects took one tablet of ethambutol hydrochloride test preparation (T) or reference preparation (R) 250 mg orally on an empty stomach in the two periods respectively, and blood samples were collected at 0 h (within 1 h before taking the medicine) and at different times after taking the medicine. After plasma was isolated, ethambutol concentrations in plasma were determined using established methods. The plasma concentration-time curve of 1 subject is shown in Figure 5 .
[0062] Based on the above examples, it can be seen that the present invention establishes a sensitive and rapid liquid chromatography-tandem mass spectrometry method for the determination of ethambutol in human plasma. Plasma samples were processed by the precipitated protein method and then directly injected for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision gas | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com