Multifunctional binder for lithium-sulfur battery positive electrode and preparation method of multifunctional binder
A lithium-sulfur battery, multi-functional technology, applied in the direction of battery electrodes, lithium batteries, non-aqueous electrolyte battery electrodes, etc., can solve the problem of volatile solvent biotoxicity, unfavorable environmental protection, inability to effectively inhibit the shuttle effect, and high boiling point that is difficult to remove. and other issues, to achieve the effect of improving cycle specific capacity and cycle stability, inhibiting the shuttle effect, and improving cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 polyrotaxane
[0059] In order to improve the reactivity of polyethylene glycol terminal hydroxyl groups, first modify polyethylene glycol to polyethylene glycol diamine, the process is: weigh 8.0g polyethylene glycol (Mn=8,000g / mol) and 0.2g Add N,N'-dicarbonylimidazole to 100mL tetrahydrofuran and react at 50°C for 18h, then weigh 0.54g of ethylenediamine and add it to the reaction system, keep it warm for 2h, add 40mL of ethanol to the product and place it in the refrigerator Precipitate after standing for 2 hours, wash the product with refrigerated ethanol, and dry for 48 hours to obtain polyethylene glycol diamine.
[0060] Weigh 10g of α-cyclodextrin and slowly add it to 20mL of deionized water, heat to 80°C, after the α-cyclodextrin is completely dissolved to obtain a transparent solution, then add 1.0g of the above polyethylene glycol diamine, keep stirring for 30min , put the mixed solution at low temperature (5-10°C) and refrig...
Embodiment 2
[0061] The preparation of embodiment 2 cationic polyrotaxane
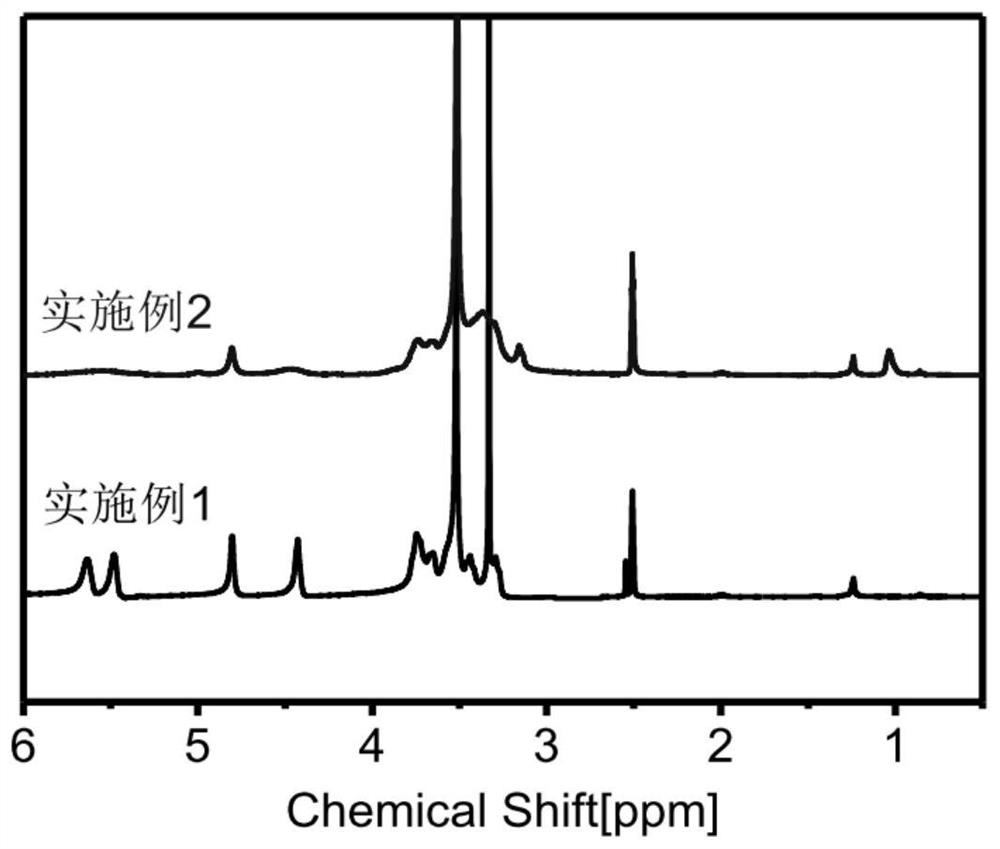
[0062] Weigh 1.0 g of the polyrotaxane obtained in Example 1 and dissolve it in 100 mL of NaOH solution with a concentration of 1 mol / L. After dissolving in an ice bath to obtain a yellow, uniform and transparent solution, add 5.0 g of 2,3-epoxypropyl tris Methyl ammonium chloride, stirred and reacted for 12 hours, the product was dialyzed with deionized water and freeze-dried to obtain a cationic polyrotaxane. cationic polyrotaxane 1 See attached for H NMR spectrum figure 1 . Depend on figure 1 It can be seen that compared with Example 1, the nuclear magnetic spectrum of Example 2 shows that the chemical shift is 3.15 in the -CH in the quaternary ammonium salt group. 3 proton signal, indicating the successful preparation of cationic polyrotaxane.
Embodiment 3
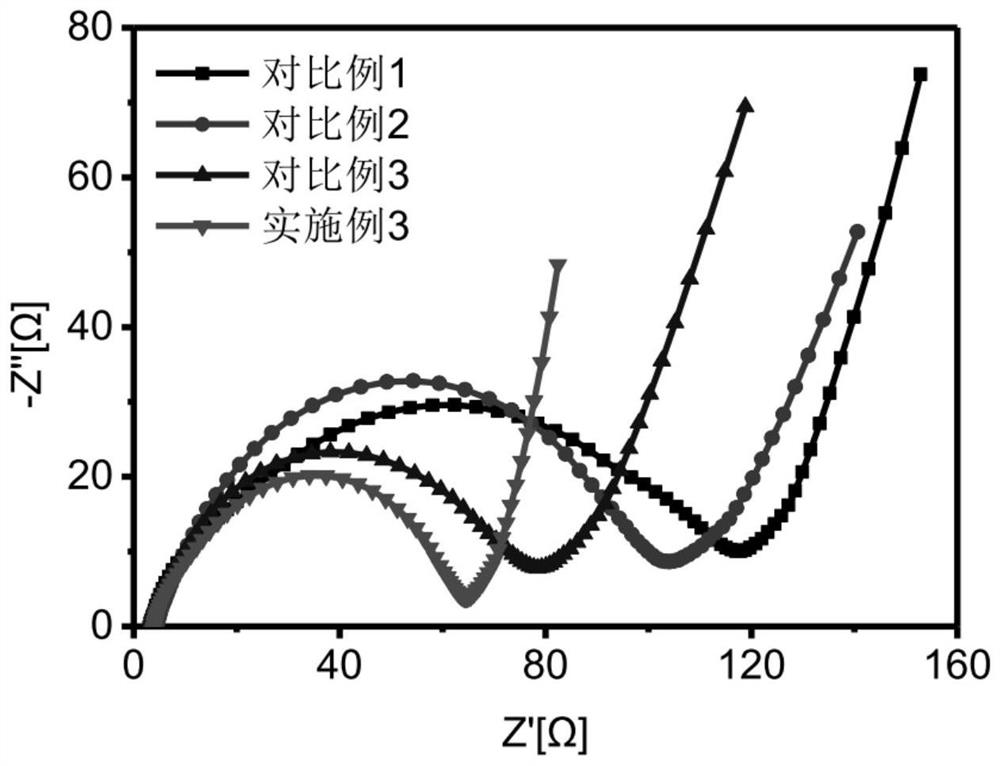
[0064] This embodiment provides a multifunctional binder for the positive electrode of lithium-sulfur battery, and provides the positive electrode of lithium-sulfur battery prepared by the multifunctional binder, and the assembled lithium-sulfur battery.
[0065] Preparation of multifunctional binder: Weigh 80 parts of polyacrylic acid (number average molecular weight 400kDa) into DMSO solvent, add 0.01 part of N,N-dicarbonyl imidazole, stir at 50°C for 12h, then weigh polyrotaxane 5 parts and 15 parts of cationic polyrotaxane were added to the above reaction solution, stirred for 72 hours, and the obtained product was a multifunctional binder for positive electrodes of lithium-sulfur batteries.
[0066] Preparation of the positive electrode: weigh the composite of sulfur / carbon black, conductive agent and binder according to the ratio of 8:1:1 by mass, add deionized water after grinding for 0.5h, stir to form a uniform slurry, and put The slurry was coated on carbon-coated al...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com