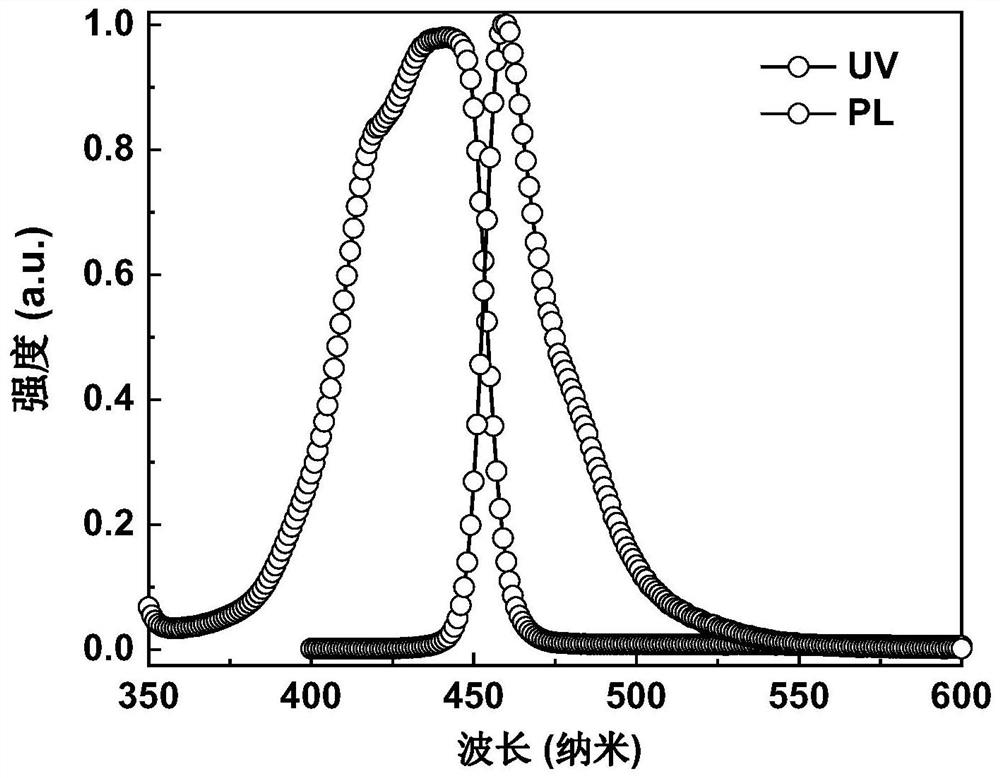
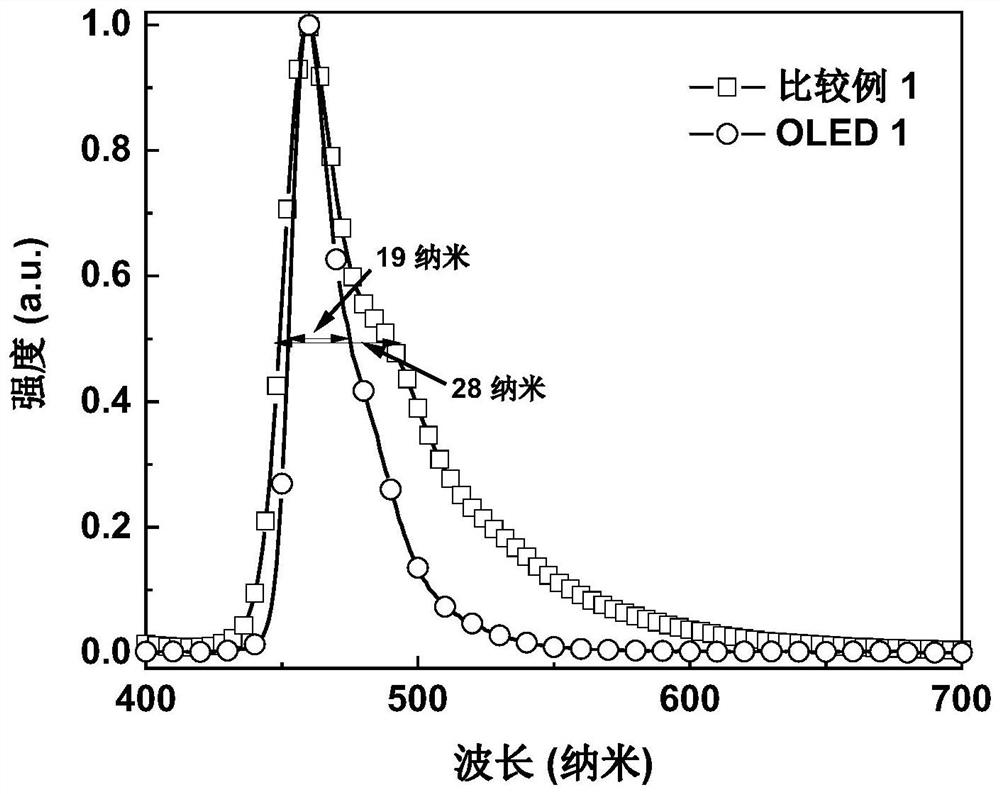
Polycyclic boron-containing compound and electronic device thereof
A boron compound and electronic device technology, applied in the field of organic optoelectronic materials, can solve the problems of difficult material purification, limited application, easy formation of isomers, etc., and achieves broad industrialization prospects, simple preparation methods, and narrow half-peak widths.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Embodiment 1: the synthesis of compound 1-5
[0147] [Synthesis of Intermediate 1-5-1]
[0148]
[0149] Under a nitrogen atmosphere, 1-chloro-9,9-dimethylfluorene (11.4 g, 50.0 mmol), 2,4,6-trimethylaniline (6.8 g , 50.0mmol), sodium tert-butoxide (9.6g, 100.0mmol), n-butylbis(1-adamantyl)phosphine (1.8g, 10.0mmol), tris(dibenzylideneacetone)dipalladium (2.9g , 5.0mmol) and toluene (200mL), and heated to reflux for 6h. After the reaction, the system was cooled to room temperature. A large amount of water was added to form a white precipitate, which was collected by suction filtration. The precipitate was washed successively with water and methanol (50% (V / V)). Finally, the resulting filter cake was dissolved in an appropriate amount of dichloromethane, and further purified by column chromatography (mobile phase: petroleum ether: dichloromethane = 3:1 (V / V)) to obtain 13.2 g of a white solid, with a yield of 80%. . MS(EI):m / z 327.21[M + ];C 24 h 2...
Embodiment 2
[0156] Embodiment 2: the synthesis of compound 1-27
[0157] [Synthesis of Intermediate 1-27-1]
[0158]
[0159] Under nitrogen atmosphere, 4-bromo-9,9-dimethylfluorene (13.7g, 50.0mmol), 2,4,6-trimethylaniline (6.8g , 50.0mmol), sodium tert-butoxide (9.6g, 100.0mmol), n-butylbis(1-adamantyl)phosphine (1.8g, 10.0mmol), tris(dibenzylideneacetone)dipalladium (2.9g , 5.0mmol) and toluene (200mL), and heated to reflux for 6h. After the reaction, the system was cooled to room temperature. A large amount of water was added to form a white precipitate, which was collected by suction filtration. The precipitate was washed successively with water and methanol (50% (V / V)). Finally, the resulting filter cake was dissolved in an appropriate amount of dichloromethane, and further purified by column chromatography (mobile phase: petroleum ether: dichloromethane = 3:1 (V / V)) to obtain 13.5 g of a white solid, with a yield of 82%. . MS(EI):m / z 327.21[M + ];C 24 h 25 N...
Embodiment 3
[0166] Embodiment 3: the synthesis of compound 1-52
[0167] [Synthesis of Intermediate 1-52-1]
[0168]
[0169] Under a nitrogen atmosphere, 4-bromo-9-(4-tert-butylphenyl)carbazole (18.9g, 50.0mmol), p-methylaniline (5.4g, 50.0mmol), sodium tert-butoxide (9.6g, 100.0mmol), n-butyl bis(1-adamantyl)phosphine (1.8g, 10.0mmol), tris(dibenzylideneacetone)dipalladium (2.9g, 5.0mmol) and toluene (200mL), and heated to reflux for 6h. After the reaction, the system was cooled to room temperature. A large amount of water was added to form a white precipitate, which was collected by suction filtration. The precipitate was washed successively with water and methanol (50% (V / V)). Finally, the resulting filter cake was dissolved in an appropriate amount of dichloromethane, and further purified by column chromatography (mobile phase: petroleum ether: dichloromethane = 3:1 (V / V)) to obtain 17.6 g of a white solid, with a yield of 87%. . MS(EI):m / z 327.21[M + ];C 29 h ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| spectroscopy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com