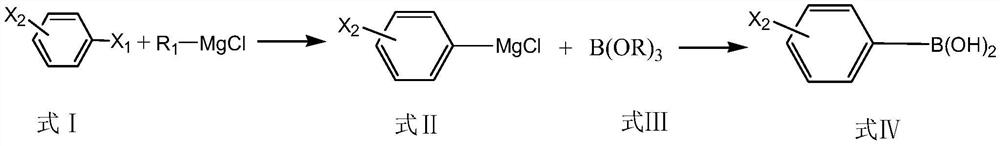
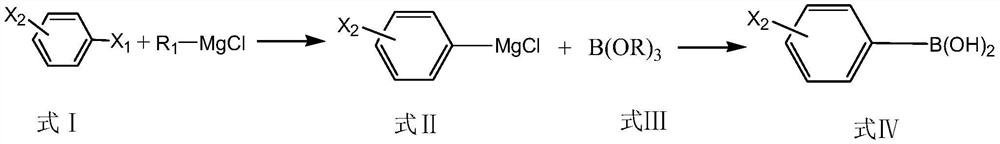
Preparation method of monohalogenated phenylboronic acid
A technology of monohalogenated phenylboronic acid and phenylboronic acid, which is applied in the field of preparation of monohalogenated phenylboronic acid, can solve the problems of harsh reaction conditions and low purity, and achieves low equipment requirements, high yield and purity, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of preparation method of 3-chlorophenylboronic acid:
[0049] Step 1. Add 47.1g (0.6mol) of isopropyl chloride to 150mL of tetrahydrofuran to obtain a solution of isopropyl chloride; put 12g (0.5mol) of magnesium chips in a 500ml three-necked flask, add 20mL of tetrahydrofuran, and set the water bath at 20°C under nitrogen Under protective conditions, stir and drop the above-mentioned isopropyl chloride solution, control the rate of addition so that the temperature does not exceed 30°C, start timing from the dropwise addition of the isopropyl chloride solution, and react for 1 hour to obtain a tetrahydrofuran solution of isopropylmagnesium chloride;
[0050] Step 2: Take 46.25g (0.25mol) of 1-butyl-3-methylimidazole formate, add 0.53g (0.0125mol) of anhydrous lithium chloride under stirring conditions, and stir until a transparent homogeneous system is obtained to obtain a catalyst; Take 4.678g of the above catalyst, add it to the tetrahydrofuran solution of isop...
Embodiment 2
[0055] A preparation method of 4-bromophenylboronic acid:
[0056] Step 1. Add 60.17g (0.65mol) of tert-butyl chloride into 150mL of methyl tert-butyl ether to obtain a tert-butyl chloride solution; put 12g (0.5mol) of magnesium chips in a 500ml three-necked flask, and add 35mL of methyl tert-butyl ether, in a water bath at 40°C, under the condition of nitrogen protection, stir and drop the above-mentioned tert-butyl chloride solution, control the rate of addition so that the temperature does not exceed 50°C, start timing from the dropwise addition of the tert-butyl chloride solution, and react for 2 hours to obtain tert-butyl ether Butyl magnesium chloride in methyl tert-butyl ether solution;
[0057] Step 2: Take 21.41g (0.125mol) of 1-butyl-2,3-dimethylimidazolium hydroxide salt, add 1.37g (0.0125mol) of anhydrous lithium sulfate under stirring conditions, stir and mix evenly to obtain a catalyst; Weigh 4.556g of the above-mentioned catalyst, add it in the methyl tert-buty...
Embodiment 3
[0061] A preparation method of 4-bromophenylboronic acid:
[0062] Step 1, 78.79g (0.7mol) chlorobenzene is added in the mixed solution of 350mL dioxane and toluene (the volume ratio of dioxane and toluene is 1:1, hereinafter referred to as mixed organic solution) to obtain chlorobenzene Solution: put 12g (0.5mol) of magnesium chips in a 500ml three-necked bottle, add 30mL of the above mixed organic solution, and put the above chlorobenzene solution in a water bath at 70°C under nitrogen protection, stirring and dropping the above chlorobenzene solution, and controlling the rate of addition so that the temperature does not exceed 80°C , start timing from the dropwise addition of chlorobenzene solution, and react for 1.5h to obtain the reaction solution of phenylmagnesium chloride;
[0063] Step 2: Take 18.52g (0.1mol) of 1-butyl-3 methylimidazole formate, add 1.74g (0.025mol) of lithium formate monohydrate under stirring conditions, and stir until a transparent homogeneous sys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com