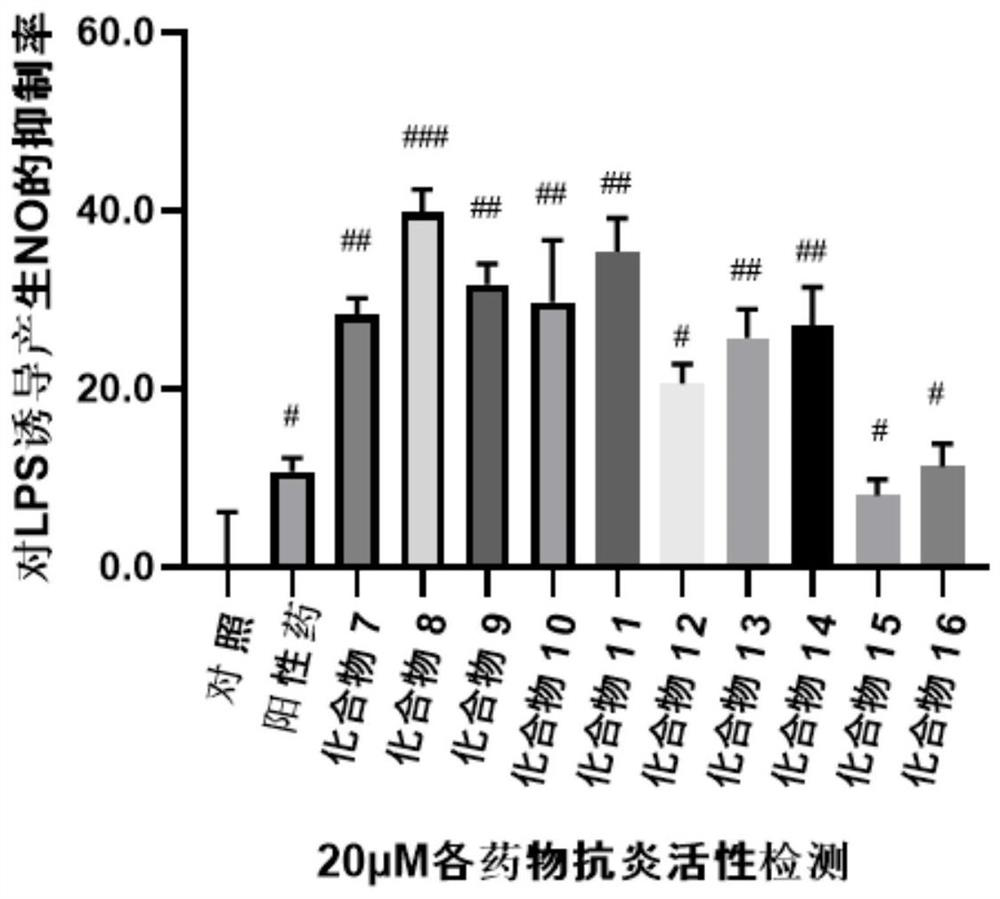
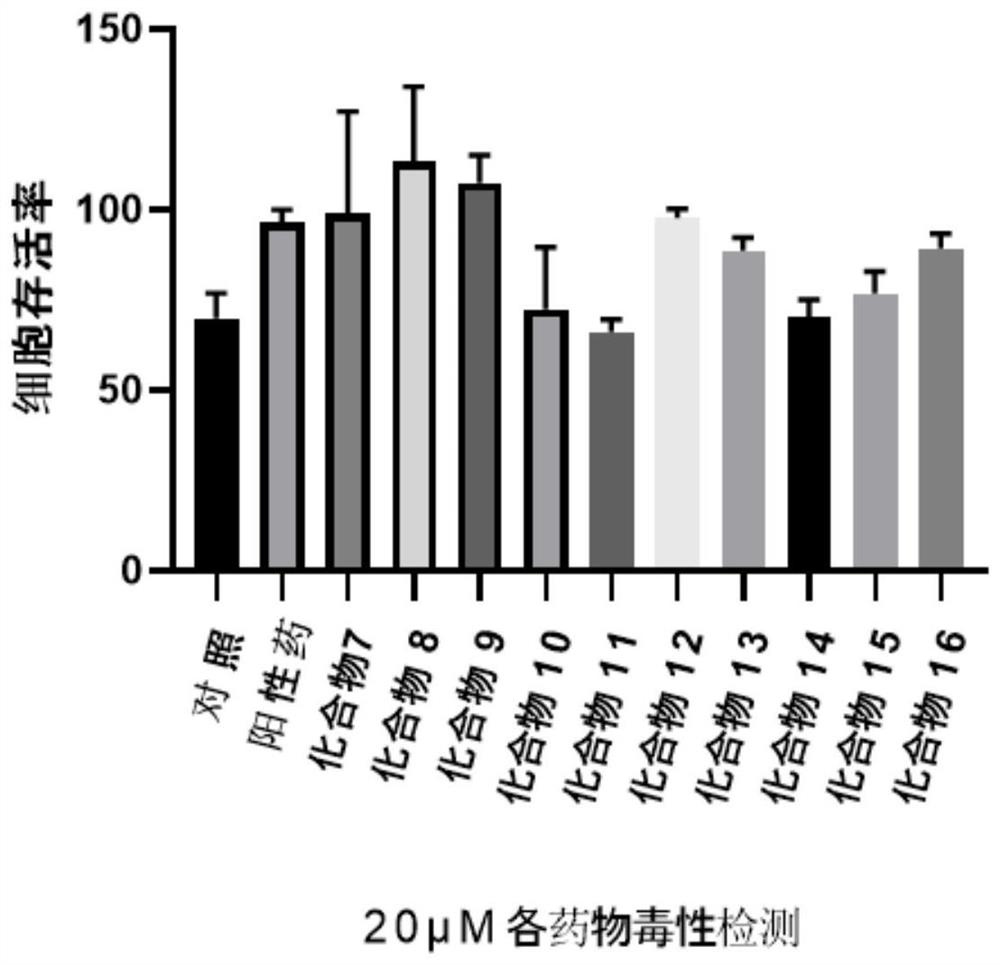
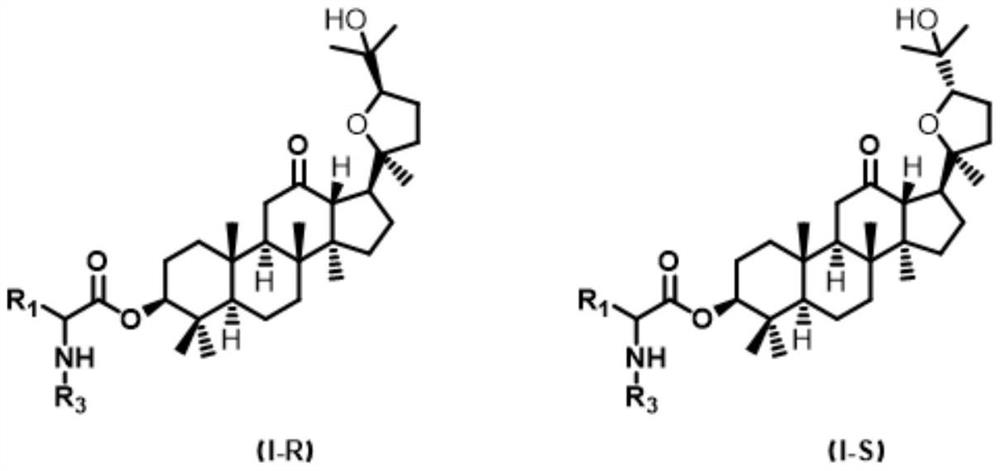
Ocotillol type esterified derivatives, preparation method thereof and application of Ocotillol type esterified derivatives in preparation of anti-inflammatory drugs
A derivative and esterification technology, which is applied in the preparation and application of new compounds, can solve the problems of shortened half-life of compounds, poor stability, and impact on drug safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: (20S,24R)-epoxy-25-hydroxy-3-ketone-dammarane-12-one
[0083] 20S-protopanaxadiol (1.000 g, 2.17 mmol) was dissolved in dichloromethane (21.7 mL), m-CPBA (549 mg, 3.18 mmol) was added, and stirred at room temperature for 3 h. Diluted with chloroform and washed with water, washed with saturated saline, dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography gave white solid compound 1 [(20S,24R)-epoxydammarin-3β,12β,25-triol] (530mg, 1.11mmol, yield 53%) and white solid compound 2 [(20S,24S)-epoxydammarin-3β,12β,25-triol] (419mg, 0.88mmol, yield 41%).
[0084] The above compound 1 (20S,24R)-epoxydammarane-3β,12β,25-triol (727mg, 1.52mmol), sodium bicarbonate (769mg, 9.15mmol) was dissolved in anhydrous dichloromethane (15mL) , add DMP (1617mg, 3.81mmol), add 2mL tert-butanol to accelerate the dissolution of DMP, react for 1 hour, remove the ice bath and react at room temperature. After reacting for 4 hours, add saturated sodium...
Embodiment 2
[0086] Example 2: (20S,24S)-epoxy-25-hydroxy-dammarane-3,12-dione
[0087]
[0088] The above compound 2(20S,24S)-epoxydama-3β,12β,25-triol (605mg, 1.27mmol), sodium bicarbonate (639mg, 7.61mmol) was dissolved in anhydrous dichloromethane (13mL) , DMP (1345 mg, 3.17 mmol) was added, and 2 mL of tert-butanol was added to accelerate the dissolution of DMP. After 1 hour of reaction, the ice bath was removed for reaction at room temperature. After reacting for 4 hours, add saturated sodium bicarbonate and sodium sulfite aqueous solution to the reaction system to adjust the pH and stir for half an hour, extract with ethyl acetate, and the organic phase is washed with saturated sodium bicarbonate aqueous solution and saturated brine respectively, and then merged into the Erlenmeyer flask , dried over anhydrous sodium sulfate, filtered and concentrated. Column chromatography (petroleum ether: ethyl acetate = 3:1 → 1:1) was eluted to obtain compound 4 (20S, 24S)-epoxy-25-hydroxyl-...
Embodiment 3
[0089] Example 3: (20S,24R)-epoxy-25-hydroxy-3β-hydroxy-dammarane-12-one
[0090]
[0091] Compound 3 (214mg, 0.45mmol), sodium borohydride (34mg, 0.91mmol) was dissolved in isopropanol (6.5mL), stirred at room temperature for 24h. The reaction was quenched with water, extracted with dichloromethane, and the organic phase was washed with anhydrous sulfuric acid Sodium-dried, filtered, concentrated, and obtained compound 5 (20S, 24R)-epoxy-25-hydroxyl-3β-hydroxyl-dammarane-12-one (180mg, 0.38mmol, yield 83.7%) by column chromatography
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com