Oligopeptide containing Hyp-Gly sequence and having antiplatelet and antithrombotic functions
An oligopeptide and sequence technology, applied in the fields of functional food and biomedicine, can solve the problems of high bleeding risk and increase bleeding events, and achieve the effects of prolonging bleeding time, easy absorption of enzymatic hydrolysis and inhibiting thrombosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1. Synthesis and functional verification of oligopeptides containing OG sequences
[0079] 1. Synthesis of oligopeptides containing OG sequence
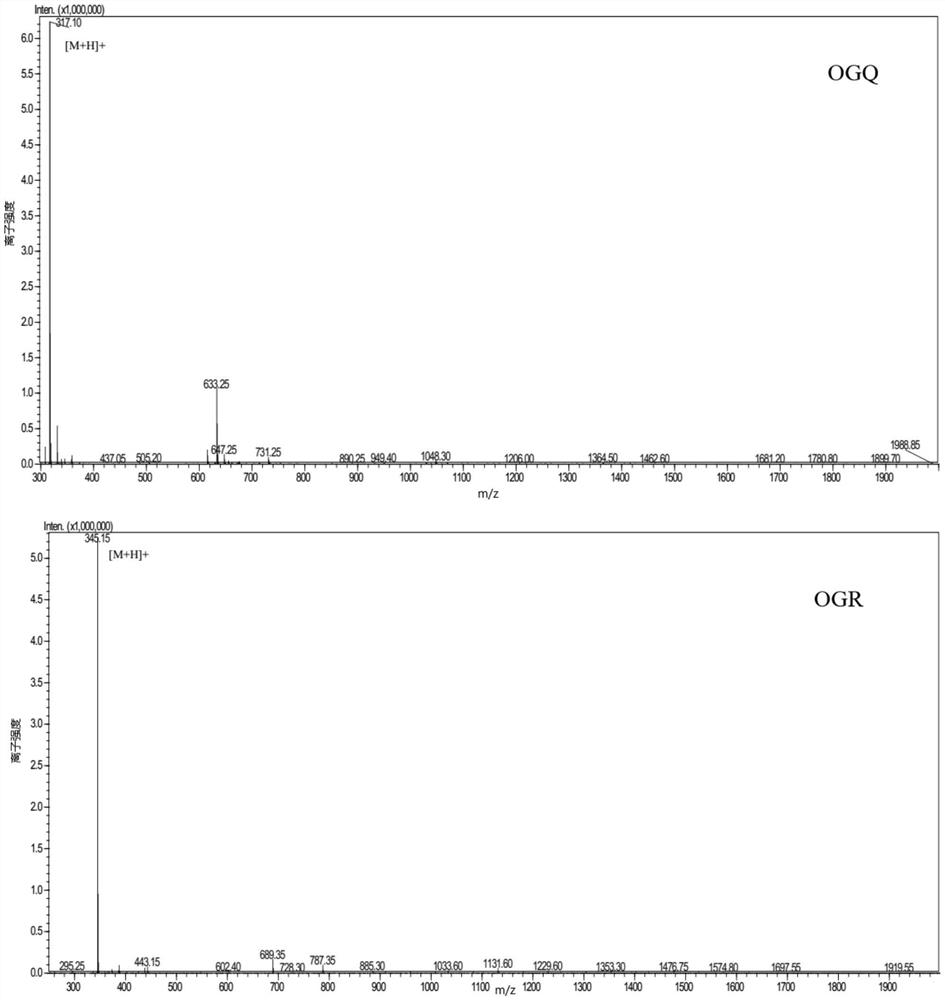
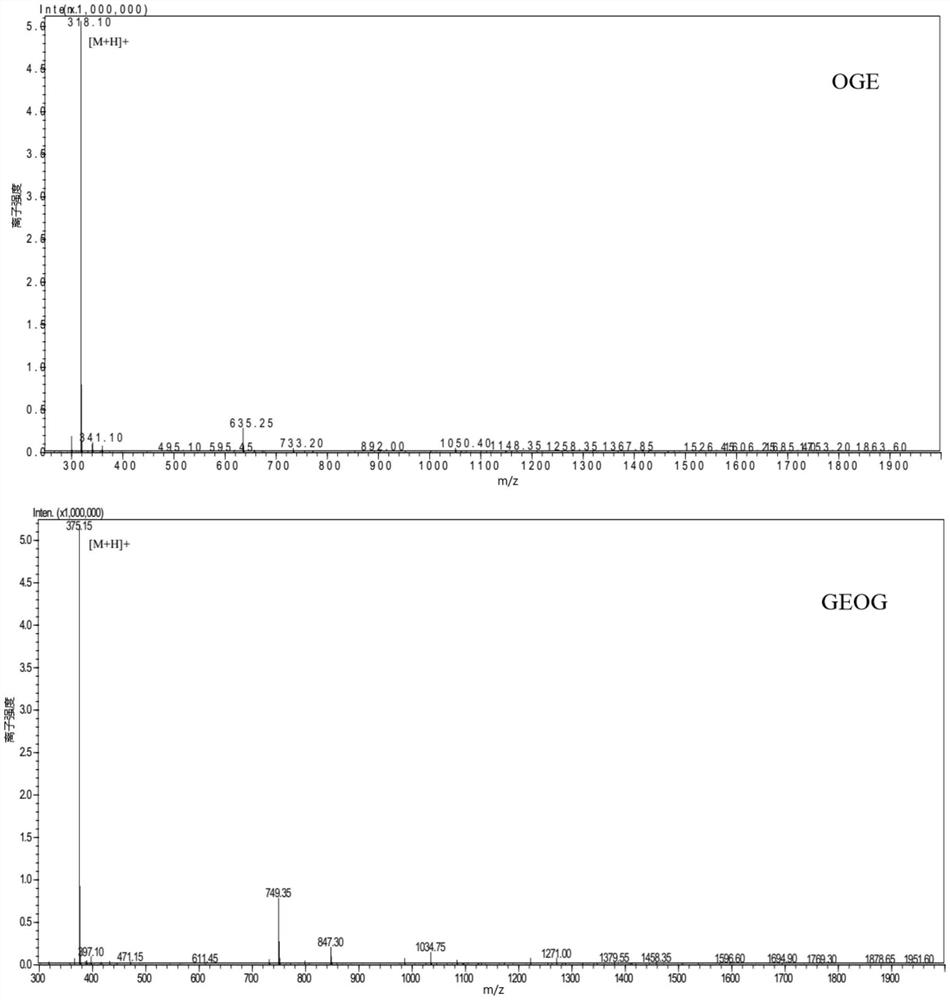
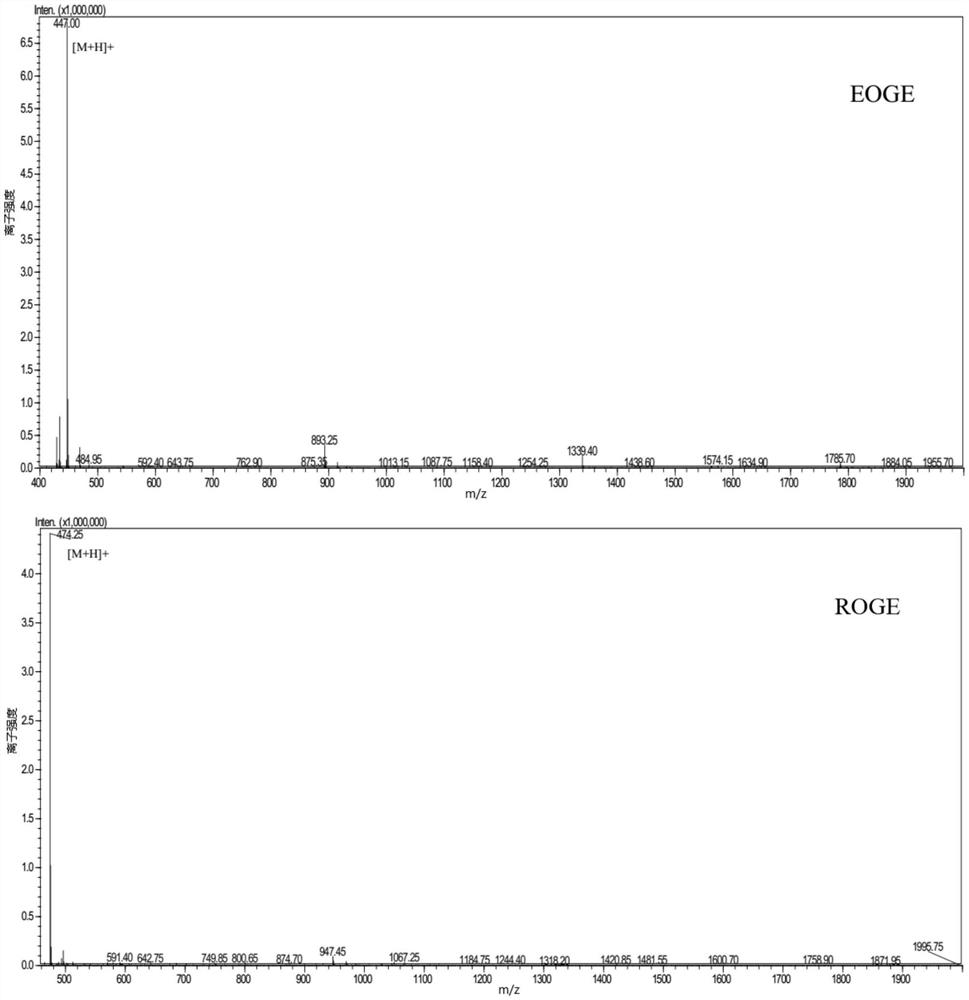
[0080] The following peptides were synthesized: Pro-Gly-Glu-Hyp-Gly-Glu (PGEOGE), Glu-Hyp-Gly-Glu (EOGE), Hyp-Gly-Arg (OGR), Hyp-Gly-Ser-Glu (OGSE), Hyp-Gly-Glu (OGE), Hyp-Gly-Ser-Ala (OGSA), Arg-Hyp-Gly-Glu (ROGE), Hyp-Gly-Gln (OGQ), Pro-Gly-Glu-Hyp-Gly ( PGEOG), Gly-Glu-Hyp-Gly (GEOG), Hyp-Gly-Hyp-Met-Gly (OGOMG), Hyp-Gly-Glu-Phe-Gly (OGEFG).
[0081] The above 12 were synthesized by "Zhejiang Hongtuo Technology Co., Ltd." by solid-phase peptide synthesis, and the purity of the compound was verified by mass spectrometry to be greater than 98%. The result is given by figure 1 shown.
[0082] The oligopeptide solution containing the OG sequence is composed of the oligopeptide containing the OG sequence and a solvent, the solvent is deionized water, and the concentration of the solution is 10 mM (the concentration o...
Embodiment 2
[0113] Embodiment 2, prepare silver carp skin antiplatelet oligopeptide
[0114] One, utilize alkaline protease and trypsin to prepare the method for silver carp skin antiplatelet oligopeptide
[0115] 1. Preparation of collagen hydrolyzate containing OG sequence oligopeptide
[0116] (1) Extraction of silver carp skin gelatin:
[0117] After the silver carp skin is thawed, remove the scales, subcutaneous fat and muscle tissue on the surface, and rinse it with tap water. Cut the fish skin into about 0.5cm 2 Small pieces of small size, soaked in 0.05mol / L NaOH aqueous solution (material-to-liquid ratio 1:6, mass w / volume v) for 1 hour to remove fat and miscellaneous proteins, after soaking, rinse with tap water until neutral or slightly alkaline properties, to obtain the pretreated fish skin.
[0118] With 0.2% (v / v volume percentage) of H 2 SO 4 Soak the pretreated fish skin in the solution (ratio of solid to liquid: 1:6, w / v) for 1 hour to make it fully swell. After soa...
Embodiment 3
[0143] Embodiment 3, preparation Atlantic salmon fish skin antiplatelet oligopeptide
[0144] One, utilize alkaline protease and trypsin to prepare the method for Atlantic salmon fish skin antiplatelet oligopeptide
[0145] (1) Extraction of Atlantic salmon skin gelatin:
[0146] Same as the one of embodiment 2.
[0147] (2) Two-step enzymatic hydrolysis method to prepare gelatin hydrolyzate:
[0148] The first step of enzymatic hydrolysis: mix the gelatin prepared in the above (1) with water according to 6% (w / v) evenly, place the mixed solution in a water bath at 90°C for 10 minutes, pasteurize it, and after cooling, obtain sterilized Post gelatin solution.
[0149] Then use 1mol / L NaOH to adjust the gelatin solution to pH = 8.0, then add alkaline protease according to the mass ratio of enzyme to substrate 2%, and enzymolyze at 60°C for 4 hours to obtain the first-step enzymatic hydrolysis solution.
[0150] The second step of enzymatic hydrolysis: adjust the pH value of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com