Avibactam intermediate compound disulfonic acid gemini quaternary ammonium salt and preparation method thereof
The technology of a gemini quaternary ammonium salt and disulfonic acid is applied in the preparation of amino compounds, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of unfavorable industrial scale-up production, complicated operation, difficult to remove solvents, etc. The effect of industrial scale-up production, simple process operation and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
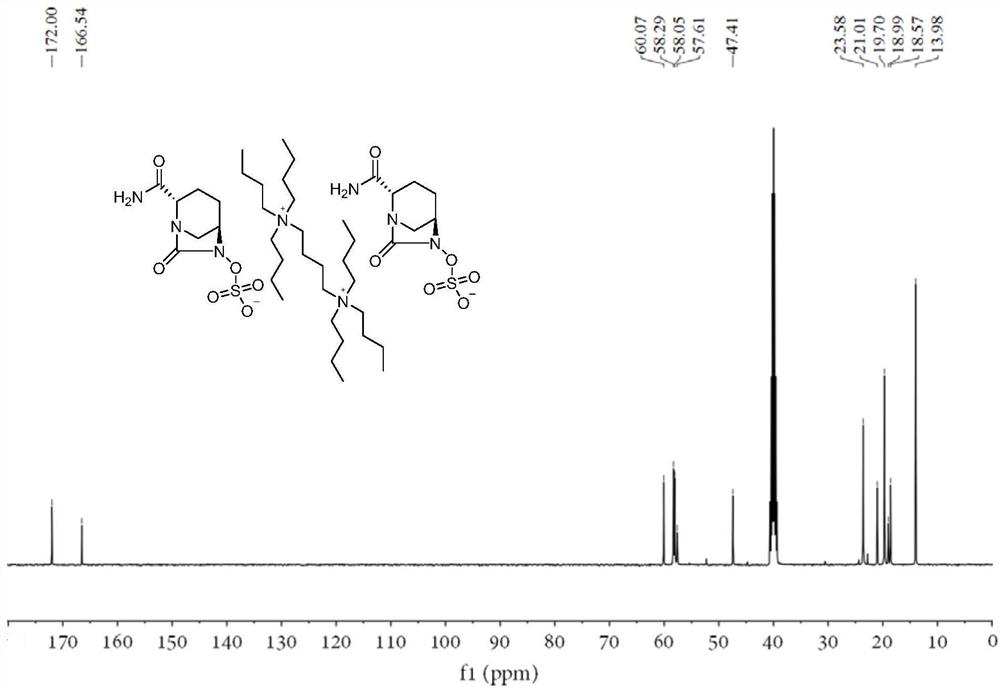
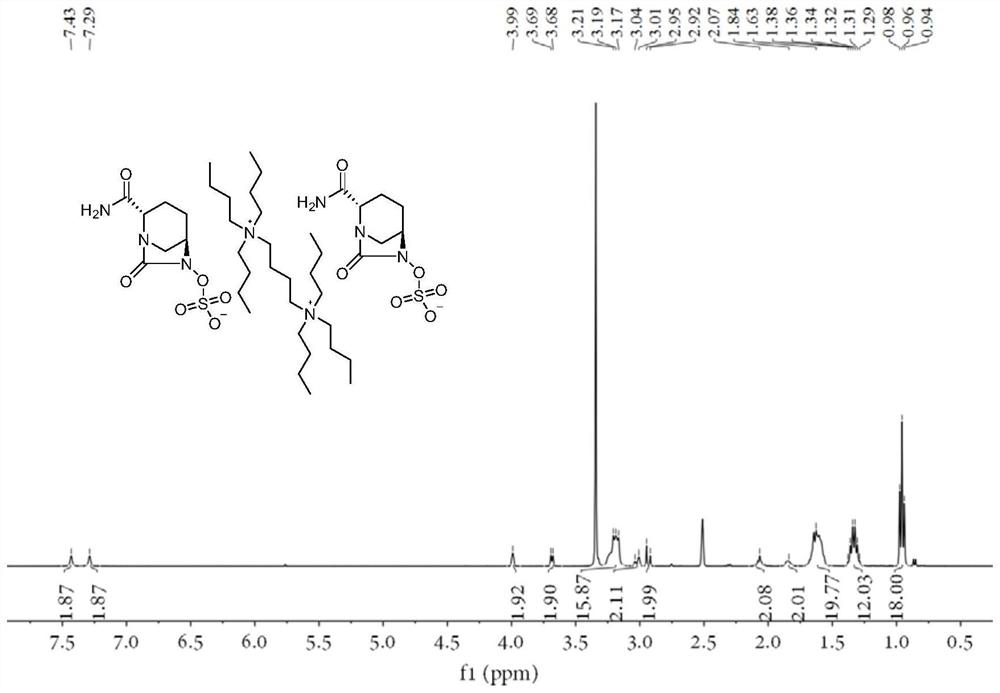
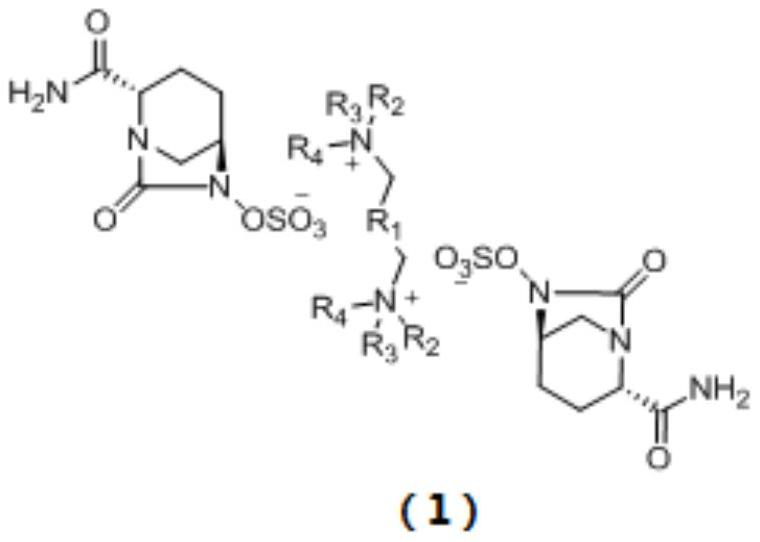
[0026] (2S,5R)-6-(Benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (2.00g, 1.00eq), 10% palladium carbon (0.05g,), sulfur trioxide trimethylamine complex (1.13g, 1.12eq), triethylamine (0.20ml, 0.2eq), isopropanol (8ml) and water (8ml) were sequentially added to 50ml In the reaction flask, hydrogen gas was introduced under the condition of stirring until the reaction was complete. Remove palladium carbon by suction filtration and wash the filter cake with water, wash the filtrate once with ethyl acetate (12ml), then add N 1 ,N 1 ,N 1 ,N 4 ,N 4 ,N 4 - Hexabutyl-1,4-butanediammonium bromide (2.60g, 0.61eq), kept at 40°C for reaction. Extract with dichloromethane (12ml×2), combine the organic phases and rotary evaporate, then add a mixed solvent of ethanol and ethyl acetate to crystallize, filter, wash and dry to obtain the avibactam intermediate disulfonic acid gemini quaternary ammonium compound 1 (2.42g, yield 70%), HPLC relative purity 99.6%.
[0027] The ...
Embodiment 2
[0029] (2S,5R)-6-(Benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (2.00g, 1.00eq), 10% palladium carbon (0.05g,), sulfur trioxide trimethylamine complex (1.13g, 1.12eq), triethylamine (0.20ml, 0.2eq), isopropanol (8ml) and water (8ml) were sequentially added to 50ml In the reaction flask, hydrogen gas was introduced under the condition of stirring until the reaction was complete. Remove palladium carbon by suction filtration and wash the filter cake with water, wash the filtrate once with ethyl acetate (12ml), then add 72% N 1 ,N 1 ,N 1 ,N 4 ,N 4 ,N 4 - Hexabutyl-1,4-butanediammonium bromide (1.87g, 0.44eq), kept at 40°C for reaction. Extract with dichloromethane (10ml), then add 28% N 1 ,N 1 ,N 1 ,N 4 ,N 4 ,N 4- Hexabutyl-1,4-butanediammonium bromide (0.73g, 0.17eq), keep the reaction at 40°C, extract with dichloromethane (10ml), combine the dichloromethane phases and rotary evaporate, add ethyl acetate The ester solvent was crystallized, filtered an...
Embodiment 3
[0032] (2S,5R)-6-(Benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (2.00g, 1.00eq), 10% palladium carbon (0.05g,), sulfur trioxide trimethylamine complex (1.13g, 1.12eq), triethylamine (0.20ml, 0.2eq), isopropanol (8ml) and water (8ml) were sequentially added to 50ml In the reaction flask, hydrogen gas was introduced under the condition of stirring until the reaction was complete. Remove palladium carbon by suction filtration and wash the filter cake with water, wash the filtrate once with ethyl acetate (12ml), then add 72% N 1 ,N 1 ,N 1 ,N 4 ,N 4 ,N 4 - Hexabutyl-1,4-butanediammonium bromide (1.87g, 0.44eq), kept at 40°C for reaction. Extract with dichloromethane (10ml), add water (4ml) and isopropanol (4ml) to the aqueous phase, add 28% N 1 ,N 1 ,N 1 ,N 4 ,N 4 ,N 4 - Hexabutyl-1,4-butanediammonium bromide (0.73g, 0.17eq), keep the reaction at 40°C, extract with dichloromethane (10ml), combine the dichloromethane phases and rotary evaporate, add ethyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com