Method for synthesizing phosphatidylserine by using immobilized biocatalyst
A phosphatidylserine and biocatalyst technology, applied in the field of biochemical science and technology, can solve the problems of deactivation, decreased reaction rate, easy aggregation and agglomeration, and achieves the effect of ensuring the quality of synthetic products, mild reaction conditions and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of immobilized phospholipase D
[0030] (1) Cut the ZnO nanowire / mesoporous silica composite carrier into small particles (less than 8mm 3 ), the carrier was immersed in an aqueous solution containing 10 mg / ml anionic cross-linker (polyethylene glycol 600), and after 30 min, the sample was taken out of the solution and washed three times with distilled water to remove free cross-linker in the water.
[0031] (2) Add phospholipase D powder to 20ml of 100mM, pH 6.0-8.0 sodium acetate buffer, keep the mixture stirring for 15 minutes, then collect the supernatant by centrifugation, and prepare PLD solutions with different concentrations (1-5mg / ml).
[0032] (3) Soak the ZnO nanowire / mesoporous silica composite carrier adsorbed with polyethylene glycol 600 in 10ml of free PLD solution with pH 6.0-8.0 for 20-30 hours, keep the temperature at 15°C, after that, The samples were washed with deionized water and sodium acetate buffer solution, and stor...
Embodiment 2
[0033] Example 2: Determination of Immobilized Enzyme Loading Capacity
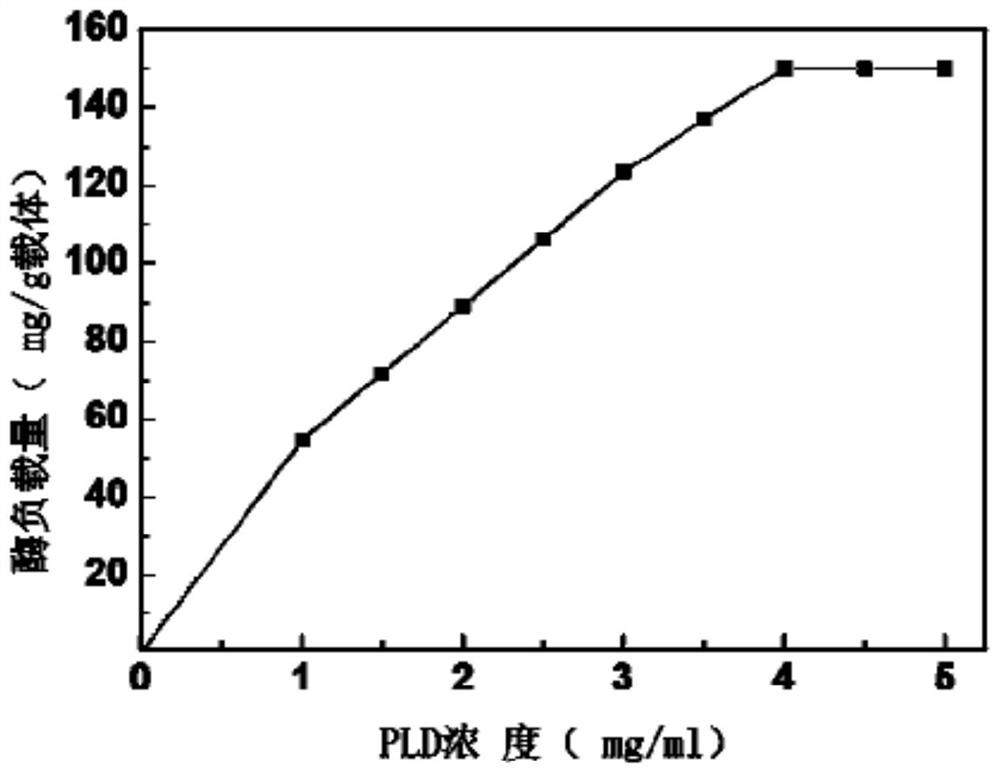
[0034] In order to measure the loading capacity of PLD in the immobilized phospholipase described in Example 1, the protein content in the enzyme liquid detects with Bradford method, uses bovine serum albumin as standard protein, draws the standard curve of protein concentration and absorbance; Initial free PLD protein concentration C 1 (mol / ml), PLD protein concentration C in the system after the immobilization reaction2 (mol / ml), PLD protein concentration in the buffer solution used to wash the sample C 3 (mol / ml); the initial volume of the reaction system V 1 (ml), the buffer volume V used to wash the samples 2 (ml), the mass of the composite carrier used is M (g), then the enzyme load per unit carrier is:
[0035] Loading capacity (mg / g) =
[0036] Experimental results such as figure 1 As shown, in the immobilization reaction process, when the free PLD concentration is 1-4mg / ml, the enzyme load i...
Embodiment 3
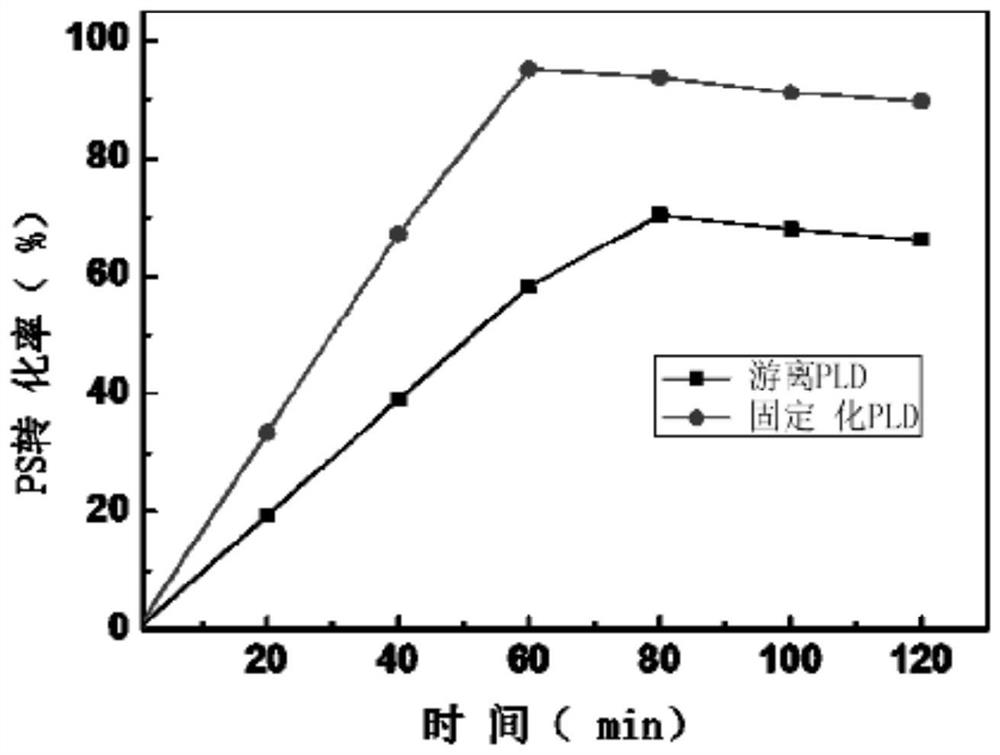
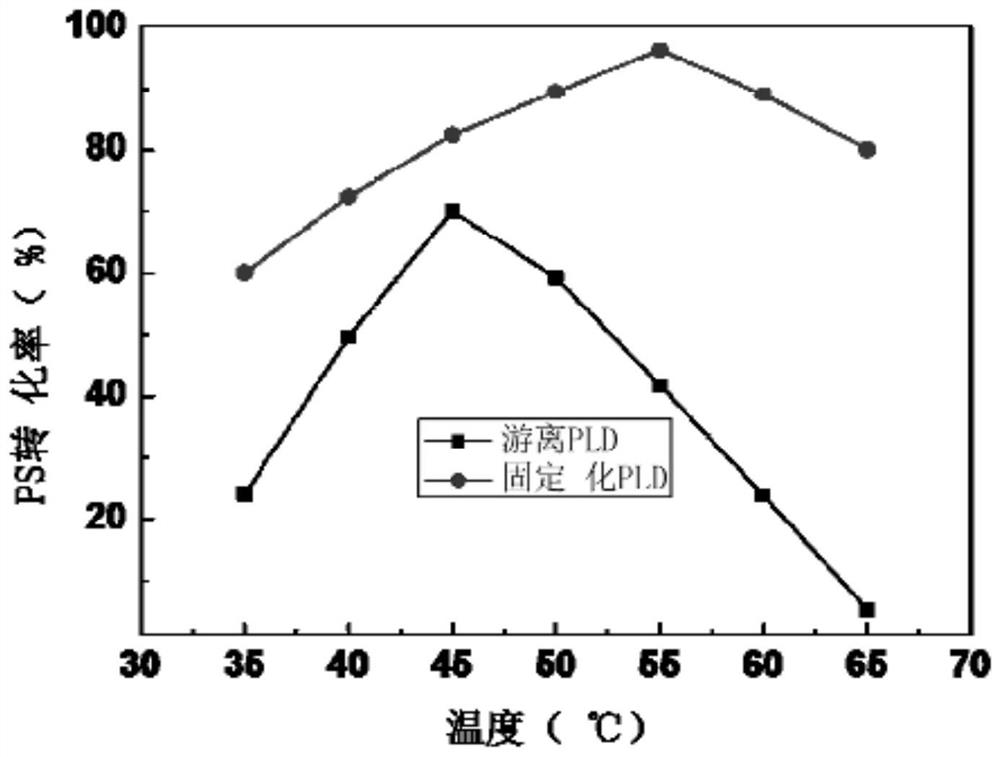
[0037] Example 3: Enzyme activity assay of free PLD and immobilized PLD
[0038] For the convenience of experiments, the hydrolysis activity of the PLD of the present invention is used to determine its activity, according to the method similar to that of D'Arrigo et al. Anal. Chim. Acta 304 (1995) 249–254.) to measure the release of nitrophenol from p-phosphatidyl-p-nitrophenol (PpNP) by PLD at 405 nm. The enzyme activity reaction system consists of 50 μl substrate solution (10mM PpNP in 10mM pH7.0 Tris / HCl, 5% Triton X-100, 5mM SDS), 400μl 0.1M pH5.5 sodium acetate buffer (containing 20mM CaCl2), and 50 μl of free PLD enzyme. After incubation at 30°C for 10 minutes, 100 μl of 1M pH 7.0 Tris / HCl (containing 0.1M ethylenediaminetetraacetic acid) buffer was added to terminate the reaction. The activity measurement of the immobilized PLD enzyme was carried out under the same conditions as the free PLD enzyme. The reaction was started with 25 mg (wet weight) of immobilized PLD, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com