A two-component cross-linked composite material applied to urology and its preparation method
A composite material and urological technology, applied in the field of medicine, can solve the problems of wound pain stimulation, difficult to remove or replace, and inelasticity, so as to improve biocompatibility, prevent inter-tissue adhesion, reduce or eliminate swelling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Obtaining of branched polylysine terminated by amino active group (first component raw material):
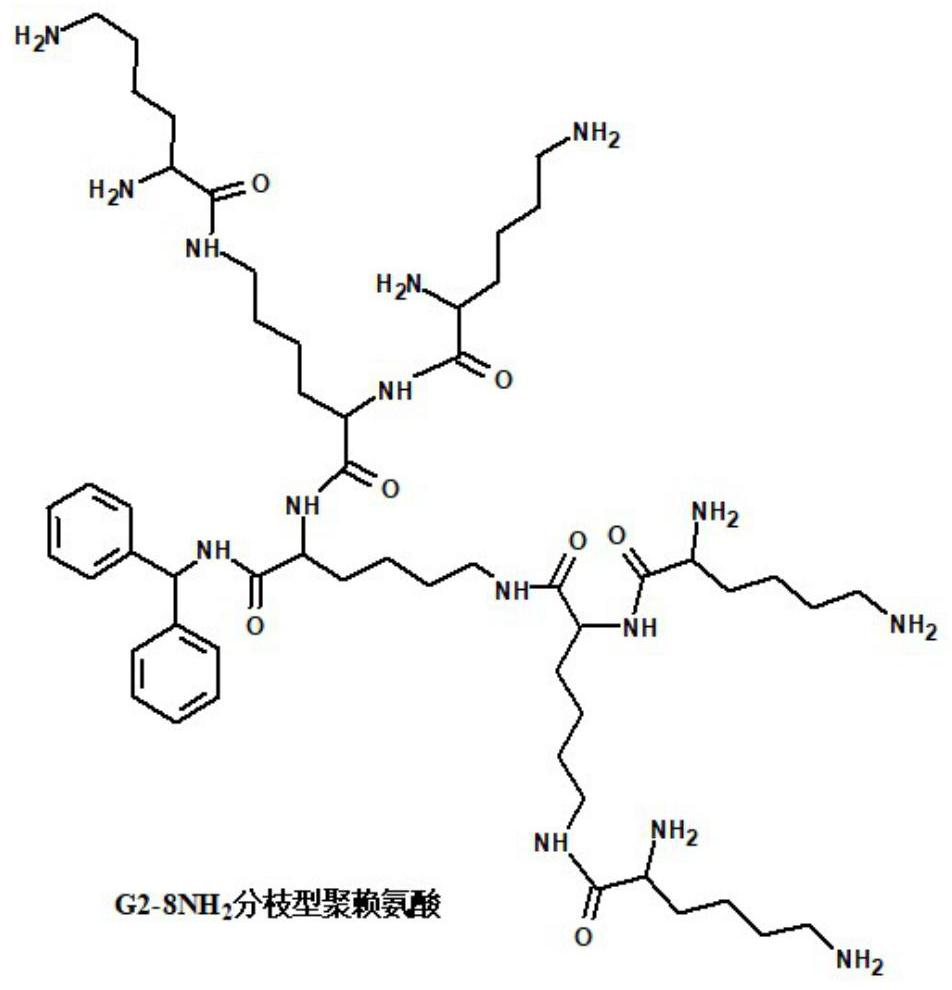
[0062] 1. G2-8NH 2 Branched polylysine has 8 branched chains, and each branched chain end contains an amino active group. The molecular structure is as follows figure 1 As shown, algebra: G2 (second generation), purchased from Weihai Chenyuan Molecular New Material Co., Ltd.
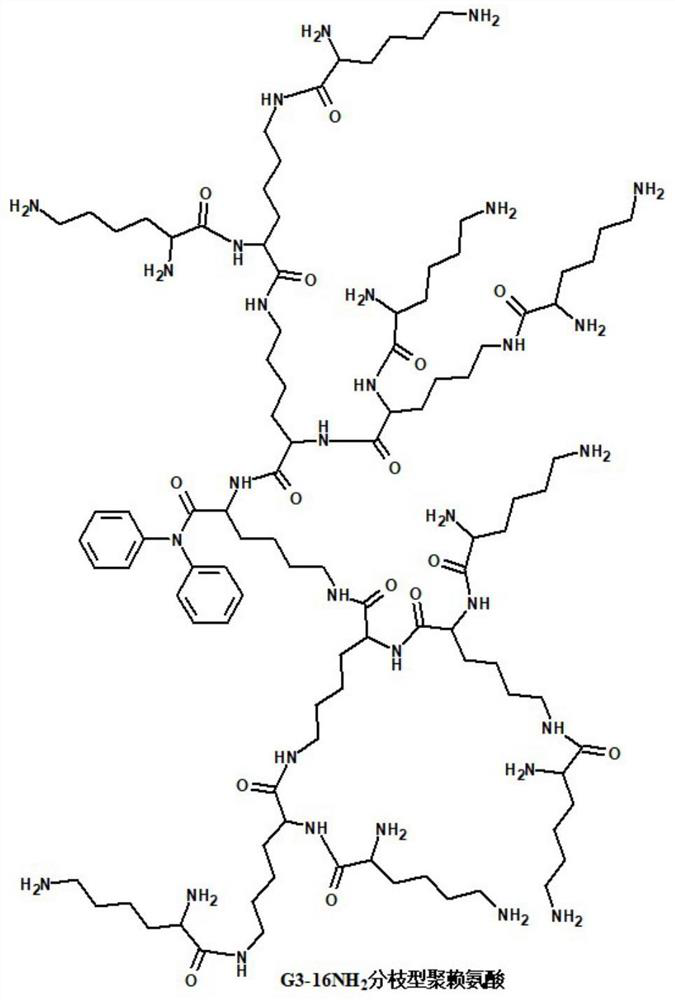
[0063] 2. G3-16NH 2 Branched polylysine, with 16 branched chains, each branched chain end contains an amino active group, the molecular structure is as follows figure 2 As shown, algebra: G3 (third generation), purchased from Weihai Chenyuan Molecular New Material Co., Ltd.
Embodiment 2
[0065] Preparation of branched polylysine terminated by maleimide active group (first component raw material)
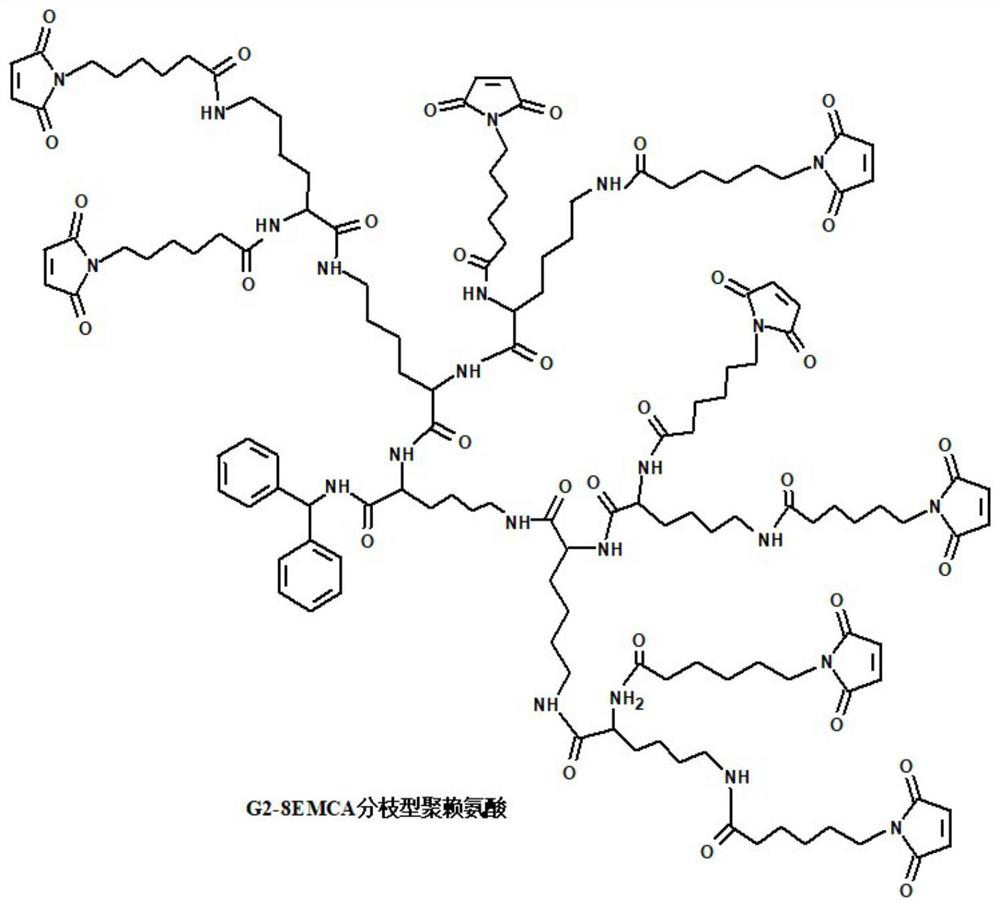
[0066] 1. Preparation of G2-8EMCA branched polylysine:
[0067] Preparation of main raw materials: G2-8NH 2 Branched polylysine, succinimidyl 6-(maleimido)hexanoate (CAS No.: 55750-63-5).
[0068] Preparation principle: first, in anhydrous organic solvent, using G2-8NH 2 The amino group at the end of the branched polylysine branch is prone to nucleophilic substitution reaction with the succinimide ester of 6-(maleimido) hexanoic acid succinimide ester, and then in G2-8NH 2 Maleimide groups were introduced into branched polylysine.
[0069] making process:
[0070](1) 60 mass parts of 6-(maleimido) caproic acid succinimide esters are dissolved in 500 mass parts of dichloromethane to form a solution;
[0071] (2) 50 mass parts of G2-8NH 2 Branched polylysine is dissolved in 500 parts by mass of N,N-dimethylformamide / dichloromethane (volume ratio 2:3) to form a so...
Embodiment 3
[0087] Preparation of branched polylysine terminated by succinimide ester active group (first component raw material)
[0088] 1. Preparation of G2-8NHS branched polylysine:
[0089] Prepare main raw material: G2-8NH of embodiment 2 2 Branched polylysine, chloroacetic acid, N-hydroxysuccinimide (CAS No.: 6066-82-6).
[0090] Preparation principle: using G2-8NH 2 -NH on branched polylysine 2 Nucleophilic substitution reaction occurs with excess chloroacetic acid to form G2-8COOH branched polylysine, and -COOH on G2-8COOH branched polylysine reacts with catalyst 1-ethyl-(3-dimethylamino Propyl) carbodiimide hydrochloride (EDC.HCl) reacts with N-hydroxysuccinimide, and then introduces succinimide ester groups on the G2-8COOH branched polylysine.
[0091] making process:
[0092] (1) 60 mass parts of G2-8NH 2 The branched polylysine is dissolved in 600 parts by mass of sodium hydroxide solution with a mass concentration of 40% to 60%, and precooled, and the precooling temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com