Method and device for preparing adiponitrile
A technology for adiponitrile and pentene nitrile is applied in the field of devices for preparing adiponitrile, and can solve the problems such as the inability to monitor the inventory content of active catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0125] The invention provides a kind of preparation method of adiponitrile, comprising:
[0126] Step 1: Hydrocyanic acid and butadiene carry out the first hydrocyanation reaction in the presence of the first catalyst to form a compound containing 3-pentenenitrile (3PN), 2-methyl-3-butenenitrile (2M3BN), The first stream of the first catalyst and butadiene, the concentration of hydrocyanic acid in the detection system is regulated by adjusting the ratio of raw materials, reaction temperature, reaction residence time or a combination of the above methods, so that the hydrogen in the final first stream is The residue of cyanic acid is less than 10ppm, and the molar ratio of the total amount of hydrocyanic acid to the amount of butadiene in the first hydrocyanation reaction is 0.75-1.0;
[0127] Step 2: the first stream obtained in step 1 is subjected to an isomerization reaction to obtain mononitriles comprising 2-pentenenitriles (2PN), 3-pentenenitriles (3PN) and 4-pentenenitri...
Embodiment 1
[0234] (1) The first hydrocyanation reaction
[0235] In the reactor R1, continuously feed the stream 101 of Cat1 (ligand: formula I, the molar ratio of total phosphorus ligand and zero-valent nickel is 12:1, impurity mass content 3.6%, 3.5Kg / h), BD The stream 102 (1.76Kg / h) and the stream 103 (0.50Kg / h) of HCN are passed into the stirred tank reactor R1 for reaction, the reaction temperature is 75°C, the reaction pressure is 2.0MPa, and the reaction residence time is 0.30h.
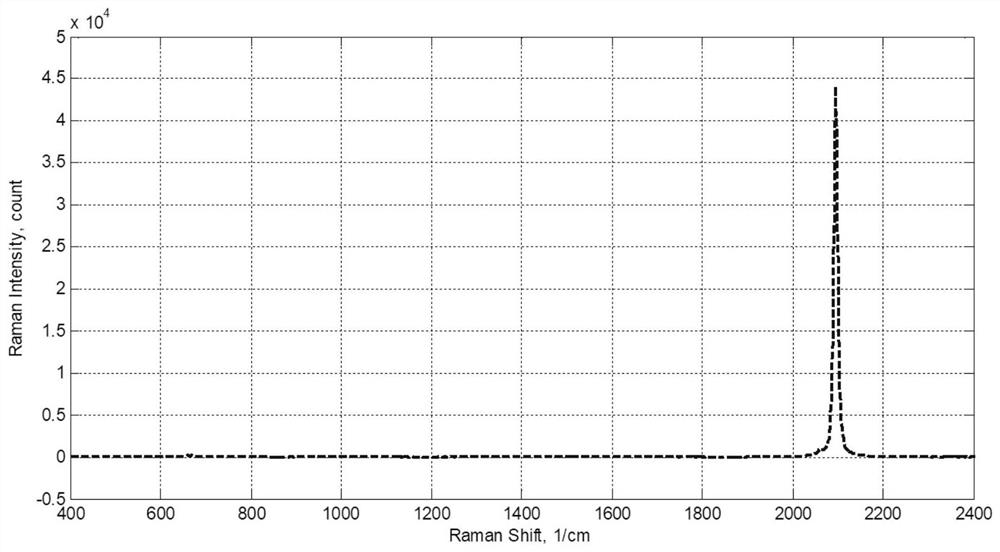
[0236] The R1 effluent stream 106 was monitored by online Raman spectroscopy, and the HCN content was 230 ppm. The stream 106 is continuously fed into the stirred tank reactor R2, and the stream 104 (0.25Kg / h) of HCN is continuously fed into R2, the reaction temperature is 75° C., the reaction pressure is 2.0 MPa, and the reaction residence time is 0.30 h.
[0237] The R2 effluent stream 107 was monitored by online Raman spectroscopy, and the HCN content was 160 ppm. The stream 107 is continuously fed ...
Embodiment 2-6
[0255] Continue the method shown in embodiment 1, reaction condition is identical, obtains following result:
[0256] Example Running time / h ADN yield / kg Cat1 loss rate / % Cat2 loss rate / % 2 10 27.1 3.9 3.0 3 50 135.5 3.8 2.8 4 100 270.9 3.8 2.7 5 200 541.8 3.8 2.7 6 500 1357.3 3.7 2.8
[0257] *The above Cat1 and Cat2 loss rates are the average loss rate per hour.
[0258]
[0259]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com