Sirolimus monoclonal antibody hybridoma cell strain and application thereof
A hybridoma cell line and antibody cloning technology, which is applied in the field of immunoassay, can solve the problems of rapid detection, cumbersome steps, and high cost, and achieve good specificity and good detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of hybridoma cell line ROX1C5
[0023] (1) Preparation of complete antigen:
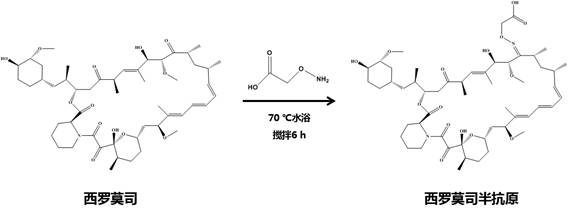
[0024] a. The hapten synthesis route is as follows:
[0025]
[0026] Weigh 19 mg of sirolimus, dissolve it in 0.6 mL of methanol:water:pyridine (volume ratio 4:1:1), add 13 mg of carboxymethoxylaminehemihydrochloride (CMO), and stir in a water bath at 70°C for 6 h , and stand overnight at room temperature in the dark. Subsequently, the solution was dried by nitrogen gas, resuspended in 1 mL of chloroform, and extracted three times with ultrapure water. The organic phase was collected and blown dry with nitrogen again, and the final product was dissolved in 1 mL of anhydrous N,N-dimethylformamide (DMF), namely the sirolimus hapten.
[0027] b. Preparation of immunogen Sirolimus-KLH: Weigh 10 mg of hapten and dissolve it in 800 μL DMF, then add 6.3 mg 1-ethylcarbodiimide hydrochloride (EDC), 5 mg N-hydroxy succinimide (NHS), the mixture was stirred at room temperatu...
Embodiment 2
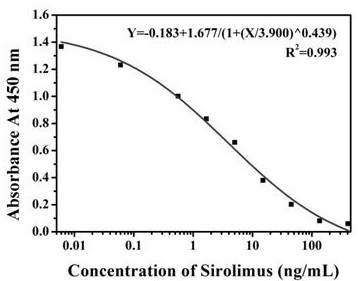
[0037] Example 2 IC of sirolimus monoclonal antibody 50 Determination of
[0038] Carbonate buffer solution (CBS): Weigh out Na 2 CO 3 1.59g, NaHCO 3 2.93g, respectively dissolved in a small amount of double distilled water and mixed, add double distilled water to about 800mL and mix well, adjust the pH value to 9.6, add double distilled water to make up to 1000mL, store at 4°C for later use;
[0039] Phosphate buffered saline (PBS): 8.0g NaCl, 0.2g KCl, 0.2g KH 2 PO 4 , 2.9 g Na 2 HPO 4 • 12 H 2 O, dissolve in 800 mL of pure water, adjust the pH to 7.2-7.4 with NaOH or HCl, and set the volume to 1000 mL;
[0040] Washing solution (PBST): add 0.5 mL of Tween-20 to 1000 mL of 0.01 mol / L PBS solution with pH 7.4;
[0041] PBST: PBS containing 0.05% Tween-20;
[0042] Antibody diluent: wash buffer containing 0.1% gelatin;
[0043] TMB Chromogenic Solution: Solution A: Na 2 HPO 4 •12H 2 O 18.43g, citric acid 9.33g, dilute to 1000 mL with pure water; solution B: dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com