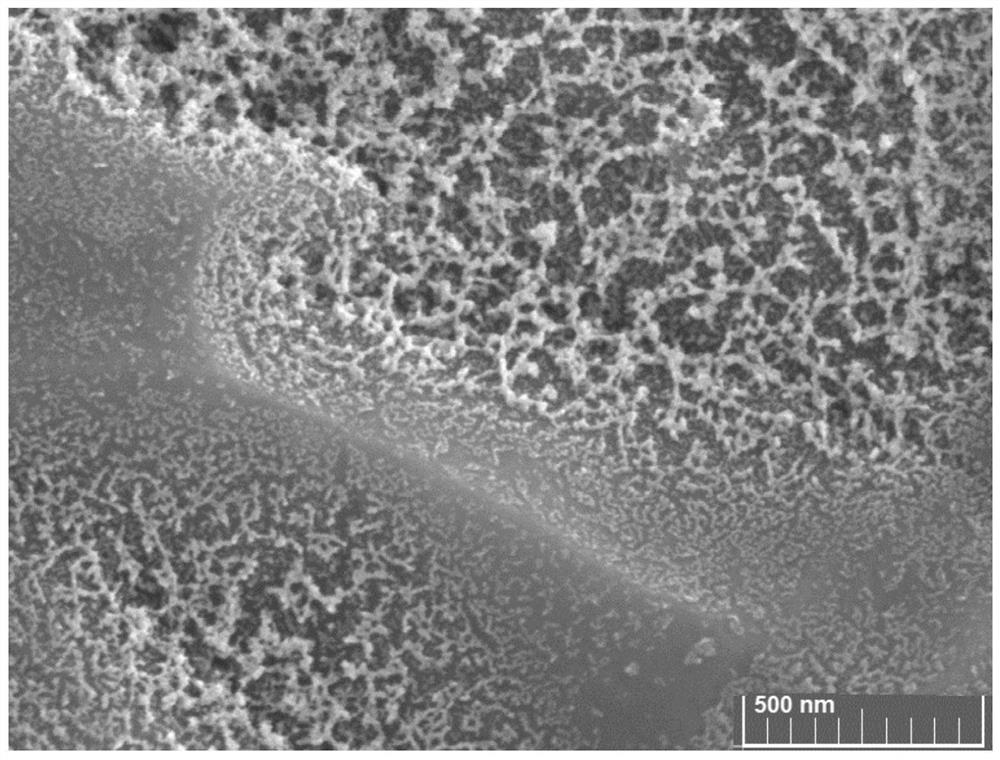
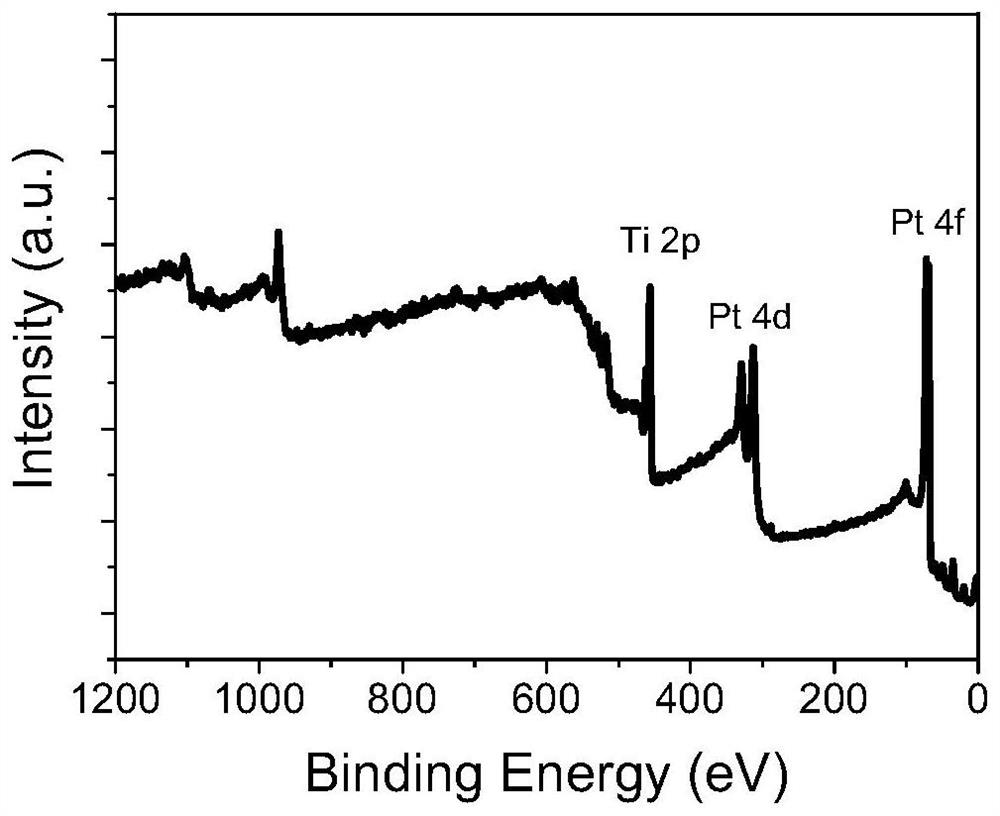
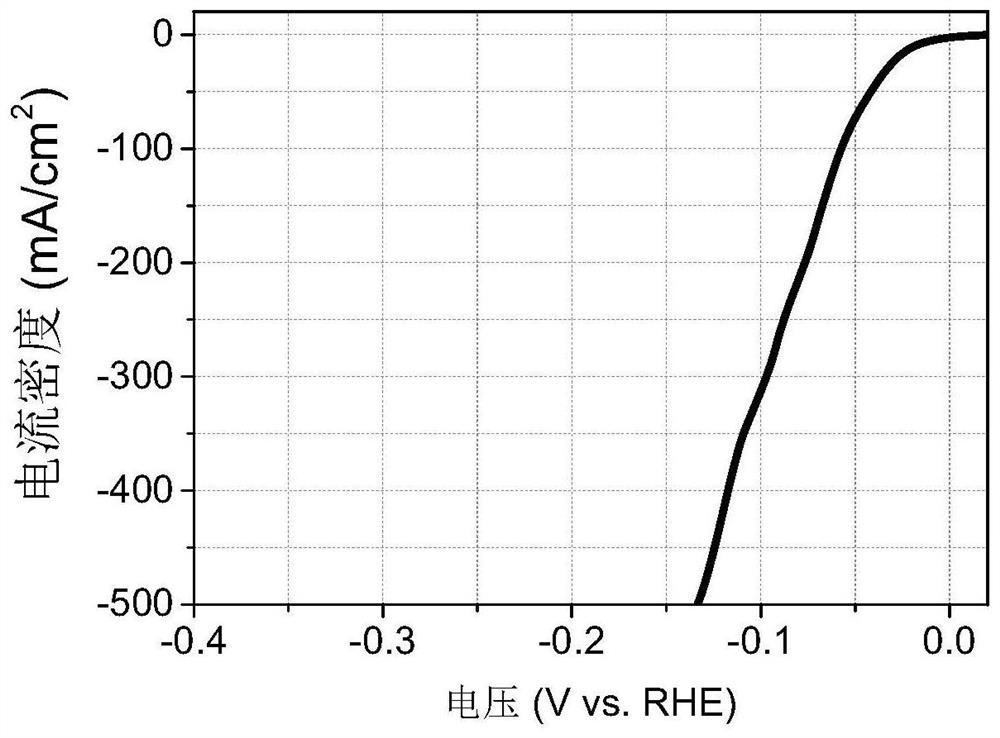
Catalytic electrode material and its preparation method and application, electrode and electrochemical cell
A catalytic electrode and electrochemical technology, which is applied to battery electrodes, circuits, electrical components, etc., can solve problems such as unevenness, catalysts that do not play a catalytic role, and thick coatings of noble metal catalysts to achieve the effect of reducing thickness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] One embodiment of the present invention provides a method for preparing a catalytic electrode material, comprising the following steps S100-S200.
[0039] Step S100, immersing the titanium mesh in a noble metal salt solution to prepare a titanium mesh loaded with a noble metal salt.
[0040] In some of the embodiments, the above soaking conditions are: soaking at -60°C to 90°C for 0.1min to 60min.
[0041] It should be noted that, in the above soaking step, there is no strict limit on the amount of the noble metal salt solution, as long as the noble metal salt solution can immerse the titanium mesh.
[0042] In some of these embodiments, the solvent of the noble metal salt solution is at least one selected from water, organic alcohols, benzene and its derivatives, furan and its derivatives, amide compounds, organic nitriles, organic ketones, alkanes and halogenated alkanes. species; the concentration of the above-mentioned precious metal salt solution is 0.01mg / mL~100m...
Embodiment 1
[0083] 1) Prepare H with a concentration of 4 mg / mL 2 PtCl 6 ·6H 2 O absolute ethanol solution.
[0084] 2) Soak the 200-mesh titanium mesh in 40wt% NaOH solution and 15wt% oxalic acid solution for 10 minutes; take out the titanium mesh and place it in the PtCl obtained in step 1). 6 ·6H 2 O absolute ethanol solution, after soaking for 10 minutes, the titanium mesh was taken out, and vacuum-dried at room temperature for 1 hour to obtain a catalytic electrode material intermediate.
[0085] 3) Place the catalytic electrode material intermediate prepared in step 2) in the furnace of the plasma equipment, turn on the plasma and pass in argon gas at a rate of 100 sccm for plasma discharge, reduce the reaction at 80°C for 10 minutes, and stop Introduce argon gas, after natural cooling to room temperature, take out the material from the plasma equipment furnace, soak and wash with deionized water, acetone and absolute ethanol in turn for 10 minutes, and finally vacuum-dry at 40°...
Embodiment 2
[0089] 1) Prepare an acetone solution with a concentration of 10 mg / mL iridium acetylacetonate.
[0090] 2) Place the 200-mesh titanium mesh successively in 40wt% NaOH solution and 15wt% oxalic acid solution for soaking treatment for 10 minutes; take out the titanium mesh and place it in the acetone solution of iridium acetylacetonate obtained in step 1), after soaking for 10 minutes, place The titanium mesh was taken out, and vacuum-dried at room temperature for 1 hour to obtain a catalytic electrode material intermediate.
[0091] 3) Place the catalytic electrode material intermediate prepared in step 2) in the furnace of the plasma equipment, turn on the plasma and pass in argon gas at a rate of 10 sccm for plasma discharge, reduce the reaction at 400°C for 15 minutes, and stop Introduce argon gas, after natural cooling to room temperature, take out the material from the plasma equipment furnace, soak and wash with deionized water, acetone and absolute ethanol in turn for 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
| power | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com