Electrode coating as well as preparation method and application thereof
An electrode and coating technology, applied in the field of electrode coating and its preparation, can solve problems such as unfavorable zinc negative electrode application, short circuit in the battery, battery failure, etc., and achieve the effects of inhibiting corrosion and hydrogen evolution reaction, super long cycle life, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Dissolve PVDF in nitrogen nitrogen dimethyl pyrrolidone solution, and then add 100nm BaTiO 3 Nanoparticles are added into the homogeneous solution, ultrasonically dispersed and magnetically stirred to make it a homogeneous slurry, in which the mass ratio PVDF: BaTiO 3 for 5:95. Use the cleaned and polished zinc foil as the base, use a coating machine to evenly coat the slurry on the zinc foil, dry it in vacuum at 60°C for no less than 3 hours, and cut it into 1cm -2 The disc electrode is used as a spare. Such as Figure 8 As shown, coated BaTiO 3 The surface of the coated zinc foil is covered with a layer of uniform nanoparticles, and further zoomed in, it is found that the coating has uniform small pores, such as Figure 9 As shown, it can be used as a channel for ion transmission. are significantly different from bare zinc foil, such as Figure 10 shown. Using XRD to characterize nano-BT found that, as Figure 11 As shown, the nanoparticles are a single BT pha...
Embodiment 2
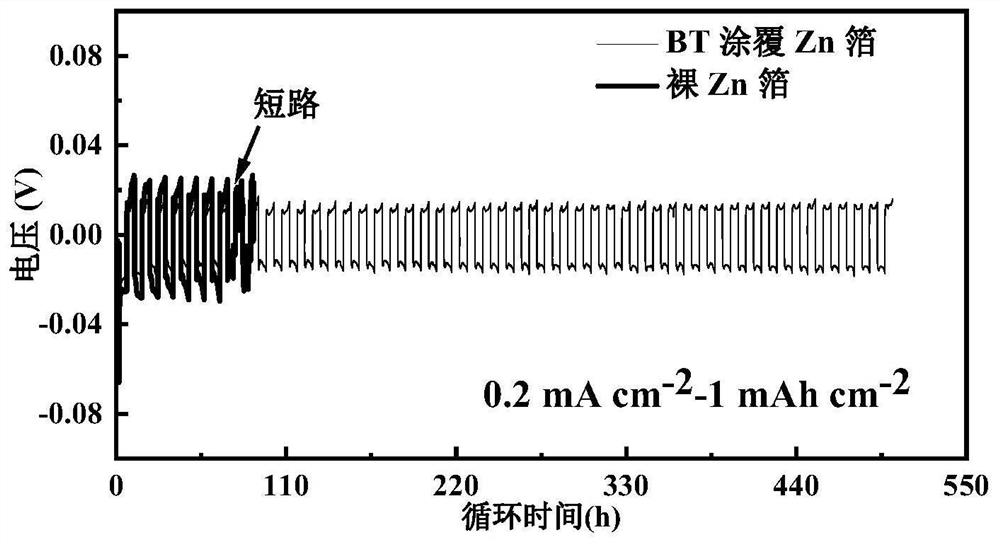
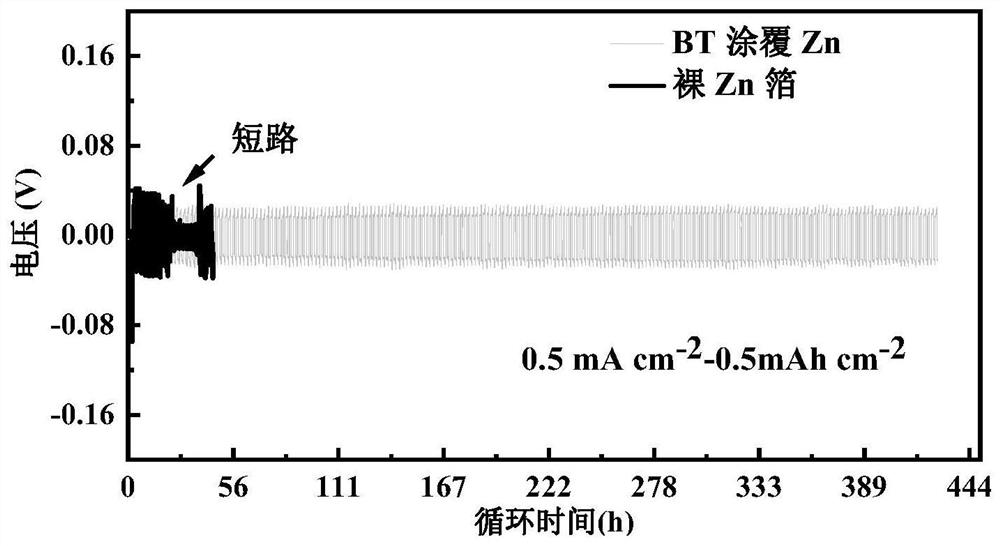
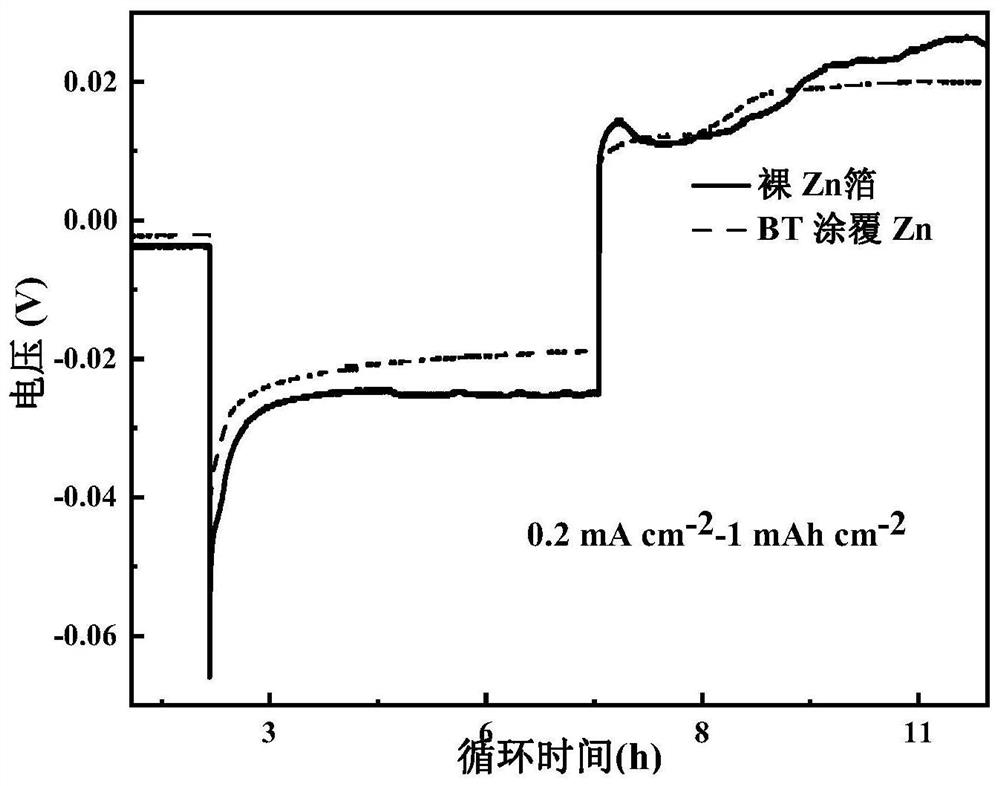
[0060] Using the BaTiO prepared in Example 1 3 The coated zinc foil was used as the electrode, and the electrolyte, diaphragm and battery case used in Comparative Example 1 were used to assemble the Zn@BT||Zn@BT symmetric battery, and the cycle stability of the battery was tested under different charge and discharge current conditions. 0.5mA cm -2 -0.5mAh cm -2 Under the same charging and discharging conditions, the symmetrical battery can stably cycle for more than 420 hours, which is 22 times that of the Zn||Zn symmetrical battery of the control example, such as figure 2 , and the Zn@BT anode has lower hysteresis voltage and nucleation overpotential, such as image 3 shown. at 0.2 mA cm -2 -1mAh cm -2 Under certain charging and discharging conditions, it can stably cycle for 500h, which is 7 times that of Zn||Zn symmetric batteries, such as figure 1 shown. Similarly, using the MnO in the control example 2 The positive electrode assembly full battery test found that ...
Embodiment 3
[0062] Use the slurry in Example 1 to evenly coat the cleaned copper foil to control the thickness of the coating, then vacuum dry at 60°C for no less than 3 hours, and cut into 1cm 2 The wafer current collector (Cu@BT). Using the electrolyte, separator and battery case in Comparative Example 1, and then using the bare zinc foil as the negative electrode, and Cu@BT to assemble a Zn||Cu@BT half-cell, the test found that the Zn||Cu@BT half-cell was 0.5mA cm -2 -0.5mAh cm -2 Under the charging and discharging conditions, it can stably cycle more than 220 times, which is 9 times that of the Zn||Cu half-cell of the control example, such as Figure 4 shown. At the same time, the study of the deposition voltage of zinc on the current collector for the first time found that the Cu@BT current collector has a lower zinc nucleation overpotential of 26mV, which is much lower than the nucleation overpotential of bare copper foil of 54mV, such as Figure 6 shown. Likewise, at 2mA cm -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| relative permittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com