Application of CD177 in preparation of product for diagnosing biliary atresia
A technology of biliary atresia and products, which is applied in the application field of products, can solve problems such as irregular arrangement of lobules, necrosis of liver cells, and poor specificity, so as to ensure the timing of diagnosis and treatment, ensure accuracy, and have strong detection specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
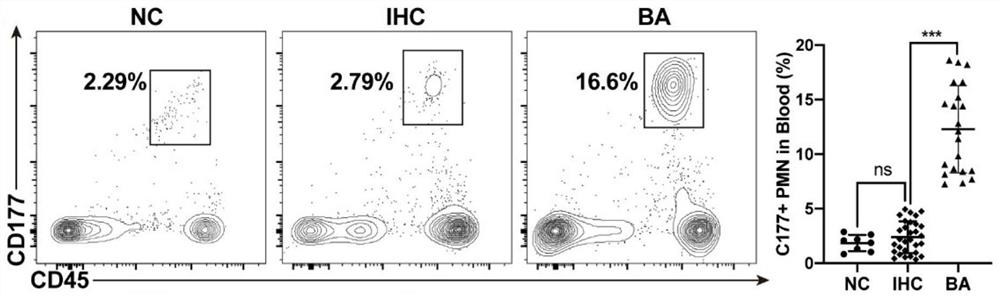
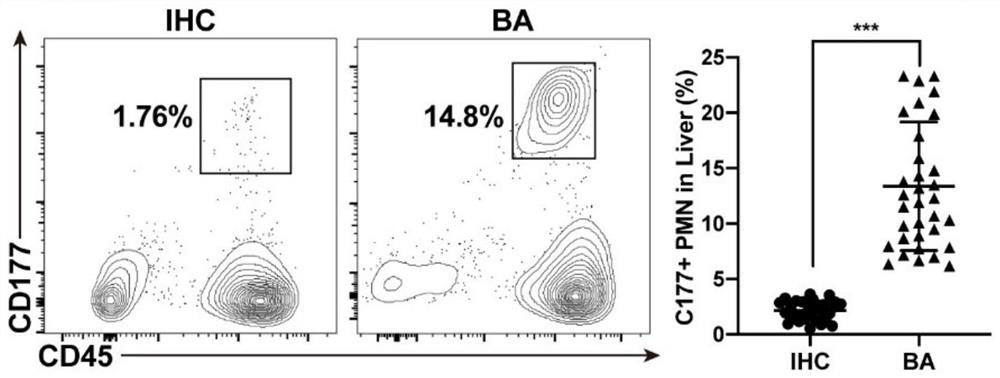
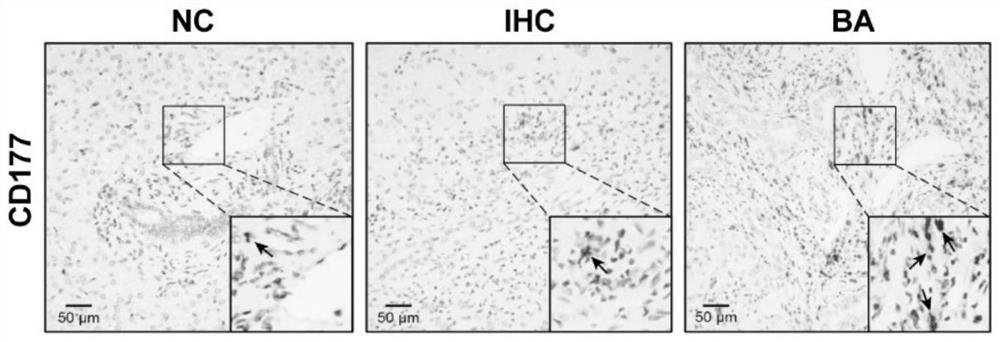
[0072] Example 1 detects the number of CD177+ cells in BA
[0073] (1) Detection of CD177+ neutrophils by flow cytometry
[0074] Clinical sample collection: 1) Liver tissue samples from children with BA and infantile hepatitis syndrome: after ethical approval and the informed consent of the patient’s family members, a fresh tissue sample from the edge of the right lobe of the liver with a diameter of about 1.5 cm was cut during biliary tract exploration or during Kasai’s operation; 2) Peripheral blood samples from children with BA, infantile hepatitis syndrome and hemangioma of the same age: after ethical approval and the informed consent of the patient's family members, fresh peripheral blood samples from children with BA, infant hepatitis syndrome and hemangioma of the same age were collected before operation and without antibiotic intervention. blood sample;
[0075] Collection of mouse liver tissue samples: Using the aforementioned method, a BA mouse model was establishe...
Embodiment 2
[0084] Example 2 further confirms the importance of CD177+ cells in BA on gene knockout mice
[0085] CD177 knockout mice were infected with RRV experiment:
[0086] (1) CD177 knockout mice (CyagenQuote: KOCMS180821DA4+KOCMS190314DA2-B) bred in the previous period were intraperitoneally injected with RRV 20 μL (titer 1.5×10 6 PFU).
[0087] (2) The mice were randomly divided into: ① only RRV injection group; ② normal mice control group. The control group was injected with the same amount of normal saline at the same time period. The liver tissue samples of mice in the above groups were collected on days 3, 6, 9, and 12.
[0088] (3) Observe the changes in the body weight, skin jaundice, survival rate and liver function of the mice; conduct extrahepatic cholangiography by routinely injecting methylene blue into the gallbladder to confirm whether there is extrahepatic biliary atresia; observe the morphology of intrahepatic and extrahepatic bile ducts in mice by pathological s...
Embodiment 3
[0090] Example 3 In vitro experiments to explore the impact of CD177+ cells on biliary epithelial cells (BEC)
[0091] 1. CD177+ cells were co-cultured with normal fetal BECs in vitro:
[0092] (1) CD177+ cell sorting in children with BA: collect peripheral blood samples from children with BA, infantile hepatitis syndrome, and hemangioma at the same age, and sort out CD177+ cells in peripheral blood by flow cytometry (see the implementation for detailed steps) example 1).
[0093] (2) Co-cultivation of sorted cells and normal fetal BECs in vitro: Place human embryonic biliary epithelial cells (BECs) in Poly-L-Lysine (PLL)-coated T75 culture flasks at 37°C, 5% CO 2 They were cultured and expanded in an incubator, and the biliary epithelial cells were purchased from ScienCell. Add the CD177+ cells from the liver or blood samples of the BA children and the disease control group extracted in the above steps to BEC medium for resuspension until the number of cells is 1×10 5 cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com