Whole-bacterium capsule as well as preparation method and application thereof
A technology for capsules and enteric-coated capsules is applied in the field of whole-bacteria capsules and their preparation, which can solve the problems of low drug efficacy, complicated process, and low concentration of bacteria, and achieve the characteristics of significant drug efficacy, reduction in the number of food types, and increase in abundance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Judgment Criteria for Healthy Donors
[0073] Feces come from healthy donors, and the selection criteria for healthy donors are as follows:
[0074] (1) Aged 18-23 years old, in good health, unmarried male or female;
[0075] (2) Regular life, no bad habits;
[0076] (3) No history of antibiotic use within three months;
[0077] (4) No gastrointestinal diseases;
[0078] (5) Both serology and fecal infectious pathogen examination and culture are negative;
[0079] (6) The types and diversity of intestinal flora are good: after high-throughput intestinal flora 16s rDNA gene detection, it is judged that the types and diversity of probiotics are good.
[0080] The exclusion criteria for healthy donors were as follows:
[0081] (1) Drug taking history: Those who have used antibiotics, laxatives, weight loss drugs or are taking immunosuppressants and chemotherapy drugs within three months;
[0082] (2) Virus exposure history: those who have been or recently e...
Embodiment 2
[0088] Example 2 Screening of fecal bacteria donors based on high-throughput sequencing
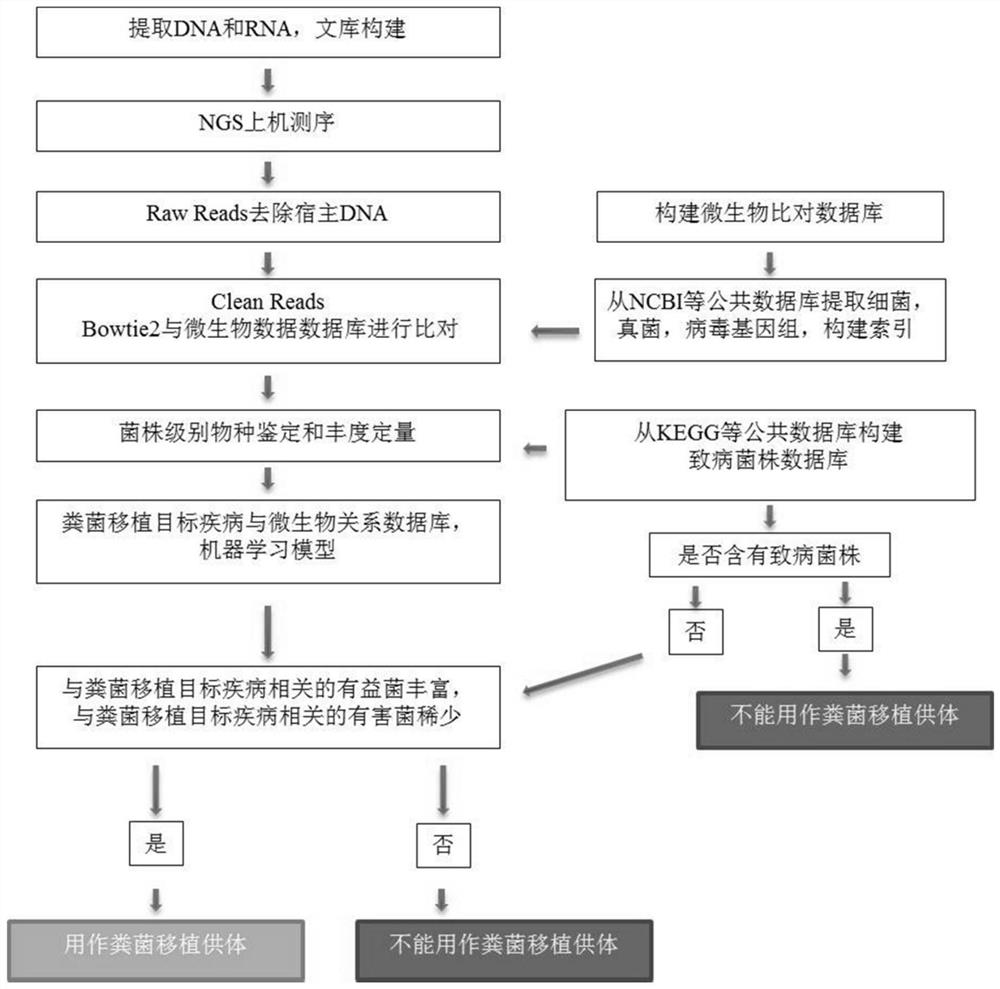
[0089] According to Example 1, people who are judged as healthy donors are used as fecal bacteria donors, and are further screened based on high-throughput sequencing technology. The flow chart is as followsfigure 1 As shown, the steps are as follows:
[0090] Extract DNA from the feces of fecal bacteria donors, construct a library for next-generation sequencing, and obtain original sequencing data; after removing the host genes of the original sequencing data, compare them with NCBI microbial databases (bacterial genomes, fungal genomes, and viral genomes). Carry out bacterial species identification and abundance detection; compare with the KEGG pathogenic bacteria database to confirm that there are no pathogenic bacteria in the fecal bacteria donor; compare with the intestinal flora of the fecal bacteria recipient to screen for complementation with the fecal bacteria recipient fecal don...
Embodiment 3
[0094] The preparation of embodiment 3 fecal bacteria liquid
[0095] Collect the feces of the fecal fungus donors screened in Example 2 on site, send them to the laboratory within 1 hour for information registration, feces identification, weighing, evaluation and treatment, and prepare the fecal fungi in an anaerobic environment solution, the steps are as follows:
[0096] (1) Soak the collected feces in sterile physiological saline at 5°C, use 2.0mm, 1.0mm, 0.5mm and 0.25mm filter screens to remove large particles, and then use 0.25mm filter screens to filter 3 times to obtain The liquid phase is fecal filtrate;
[0097] (2) The feces filtrate was centrifuged at 3000r / min for 10 minutes, and the precipitate was mixed with sterile saline to obtain a feces liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com