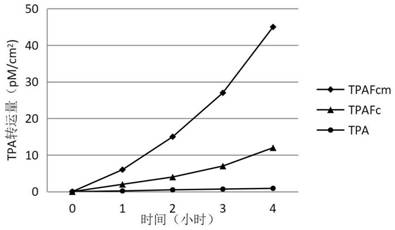
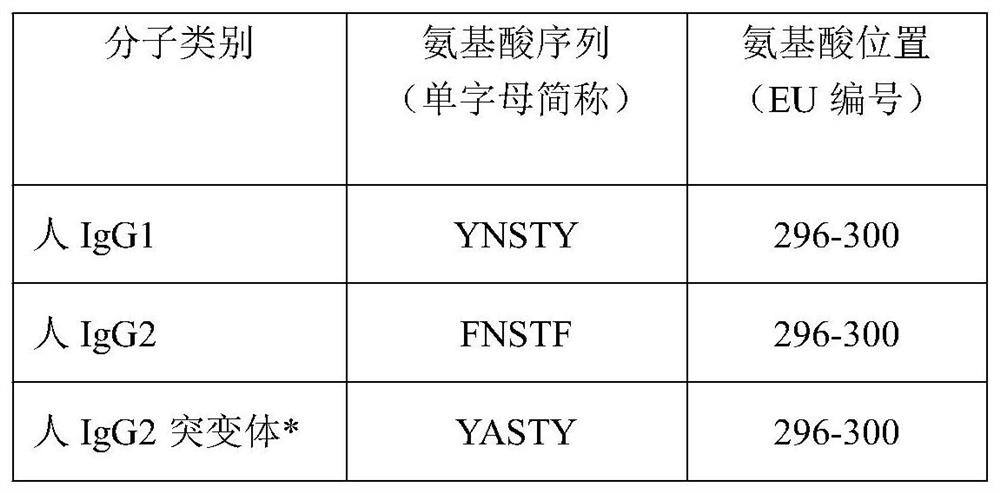
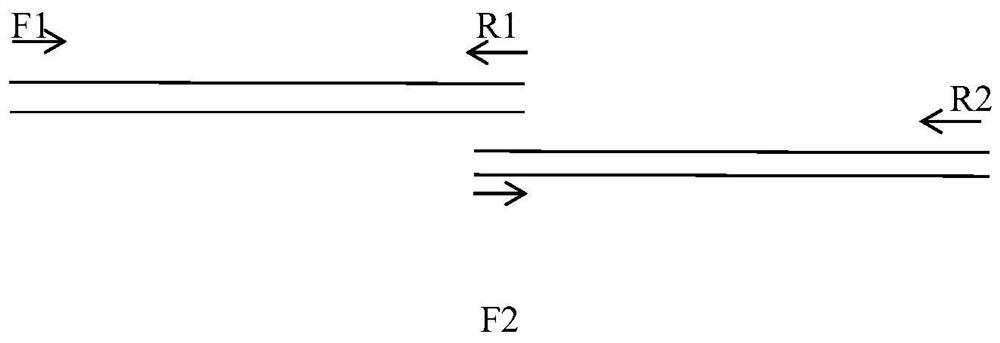
TNK-tPA fusion protein with enhanced transepithelial cell transport capacity, and application thereof
A fusion protein and epithelial cell technology, applied in the treatment and/or prevention of human vascular embolism drugs, TPAFcm fusion protein, TNK-tPA fusion protein field, can solve problems such as difficult to meet needs, and achieve improved transport capacity , change the effect of enhancement, great flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Construction and cloning of IgG2 Fc mutant gene
[0038] The human antibody IgG2 Fc fragment encoding gene used in the present invention is obtained by conventional gene cloning techniques, that is, the RNA of normal human peripheral blood leukocytes is extracted, transcribed into cDNA molecules, and then PCR-amplified with specific primers to obtain specific fragments. This fragment was cloned into the pUC18 plasmid to obtain the recombinant plasmid pUCIgG2, and its DNA sequence was confirmed by gene sequencing. It was confirmed by DNA sequencing that the cloned human antibody IgG2 Fc fragment coding gene had 100% homology with the human IgG2 antibody cDNA sequence numbered AK130614.1 in GenBank (GenBank) (such as SEQ ID NO: 1, SEQ ID NO: 2 shown).
[0039] In order to obtain the IgG2 Fc mutant gene, site-directed mutagenesis was performed by partial overlapping gene amplification technique. There are four oligonucleotide primers used in PCR amplification,...
Embodiment 2
[0045] Example 2: Construction of TNK-tPA and IgG2 Fcm fusion coding gene and recombinant expression vector
[0046] In order to construct the fusion coding gene of TNK-tPA and IgG2 Fcm, gene recombination was carried out by overlapping gene amplification technology. 所使用的寡核酸引物共有4条,分别为F3(5’gactctagaccaccatggatgcaatgaagaga3’),R3(5’tgagctgtctcggcggcggaggcctcggtggtggtgggccagcgtacaacagtgctta3’)F4(5’cgaccgggtggtggtggctccggaggcggcggctctgtcgagtgcccaccgtgccca3’),R4(5’taagtcgactcatttacccggagacag3’)。 The F3 primer contains an endonuclease XbaI recognition site (tctaga), a kozak sequence (ccaccatgg) and an initiation codon (atg), and the R4 primer contains an endonuclease SalI recognition site (gtcgac) and a stop codon. The complementary sequences of the primers R3 and F4 contain a flexible linker coding sequence (ggtggtggtggctccggaggcggcggctct) (shown in SEQ ID NO: 5, SEQ ID NO: 6).
[0047] The operation process is as follows:
[0048] The pCD4mtPA recombinant plasmid contains the codi...
Embodiment 3
[0054] Example 3: Expression of pCDTPAFcm and pCD4TPAFc recombinant expression plasmids in CHO cells
[0055] The mammalian cell expression system selected in the present invention is Chinese hamster ovary cells (CHO) deficient in dihydrofolate reductase (dhfr). The recombinant expression vector pCD4 contains an expressible dhfr coding gene. After the plasmid is transfected into CHO (dhfr-) cells, the copy number is greatly increased under the pressure of methotrexate (MTX), and at the same time, it drives the high-efficiency expression of the cloned foreign gene.
[0056] After the recombinant expression plasmids pCDTPAFcm and pCD4TPAFc constructed in Example 2 were purified and endotoxin removed, they were transfected into CHO (dhfr-) cells by liposome method, cultured in selective medium and detected by ELISA, and positive expression of TPA was screened cell line. For these positive cell lines, the concentration of MTX in the medium was gradually increased, and the highly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com