Method for preparing glycyrrhetinic acid through biological catalysis of novel glucuronidase
A technology of glucuronidase and immobilized enzyme catalyzing licorice, applied in biochemical equipment and methods, glycosylase, enzyme and other directions, can solve problems such as unfavorable industrial application, impure final product, cleavage of glycyrrhizic acid, etc. The effect of increasing difficulty and workload, reducing production costs and economical savings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
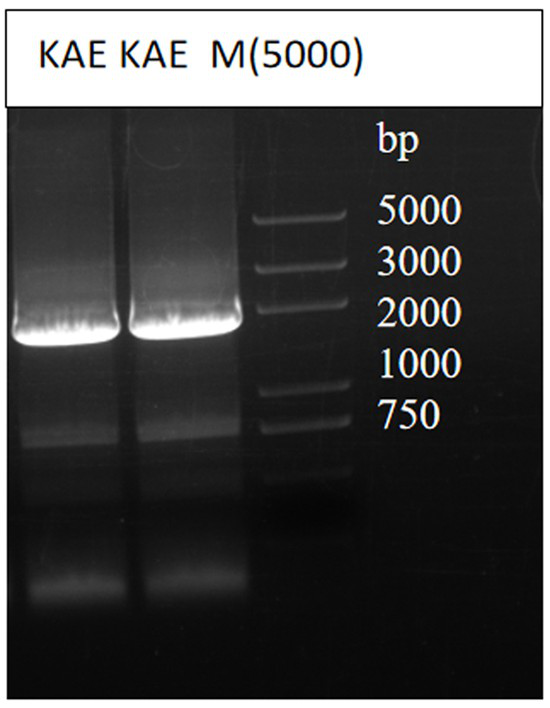
[0032] This example provides the screening and expression of β-glucuronidase. The specific method is as follows: firstly, through the gene database NCBI, compare the sequences of the existing β-glucuronidases that are widely used, and screen out a β-glucuronidase from primary The β-glucuronidase of Aspergillus fungi (85% amino acid sequence homology), gene number KAE8374379.1, the target gene was obtained by PCR amplification (such as figure 1 shown), the primers are:
[0033] Primer KAE-F: 5'-GGGAAAGGATCCATTCCGACCGATGCGCAGA-3', the enzyme cutting site is BamHI;
[0034] Primer KAE-R: 5'-GGGAAAGTCGACCTGGCACGCCTGGCCTTC-3', the enzyme cutting site is SalI.
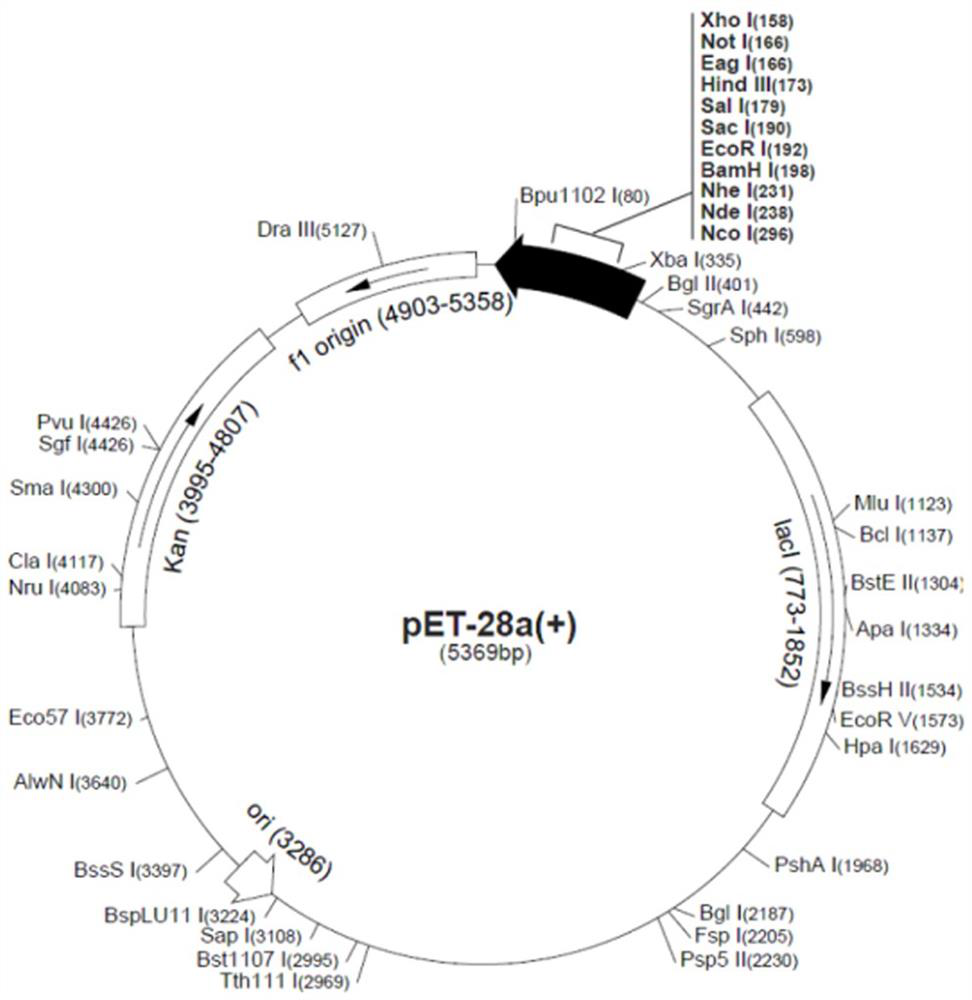
[0035] Then, the target gene was connected with the expression vector plasmid peT28a by restriction enzyme ligation (such as figure 2 , image 3 shown), introduced the expression bacteria-Escherichia coli BL21(DE3), cultured in 100mL LB liquid medium, added the inducer IPTG at OD0.6, induced at 16°C for 24h, centrifuged,...
Embodiment 2
[0037] This example constructed the immobilized enzyme Fe 3 o 4 @Zr-2MIm@KAE, the specific method is:
[0038] 1) Expression vector Fe 3 o 4 Build by @Zr-2MIm
[0039] Will Fe 3 o 4Microspheres (100 mg) were added into a methanol solution (50 ml, 1 mg / mL) of PVP, sonicated in an ultrasonic instrument for 60 min, and the supernatant was removed by adsorption with a magnet. The precipitate was collected and dispersed in a solution of 2-methylimidazole (2-MIm) in methanol (25 ml, 40 mm) under sonication. Then, Zr(NO 3 ) 4 ·5H 2 O in methanol (25 ml, 40 mm) was added to the solution. After standing for 10 minutes, collect it with a magnet and rinse it thoroughly with methanol solution. The precipitate was dried at 80 °C for 8 h.
[0040] 2) Immobilized enzyme Fe 3 o 4 Build of @Zr-2MIm @KAE
[0041] Get the crude enzyme solution of β-glucuronidase prepared in Example 1, 2mL per tube, subpackage in centrifuge tubes, add 3mg of carrier Fe constructed in each tube 3 o...
Embodiment 3
[0044] This embodiment utilizes the immobilized enzyme Fc constructed in Example 2 3 o 4 @Zr-2MIm@KAE catalyzes the preparation of glycyrrhetinic acid from glycyrrhizic acid, and the reaction schematic diagram is as follows Figure 5 As shown, the specific method is:
[0045] Weigh 10mg of glycyrrhizic acid, dissolve in 5ml pH5.0 20mM acetic acid-sodium acetate buffer, take 1ml of glycyrrhizic acid solution, add the immobilized enzyme Fe that absorbs the supernatant phosphate buffer 3 o 4 In @Zr-2MIm@KAE, react in a shaking table at 60°C for 2 hours. After 2 hours, centrifuge at 12000rpm to separate the supernatant, and the precipitate is the product glycyrrhetinic acid.
[0046] Dissolve the resulting precipitate in 1 mL of methanol as Figure 6 As shown, HPLC (methanol: 0.2% phosphoric acid 90:10, wavelength 254nm, flow rate 1mL / min, column temperature 35°C) detects the content of glycyrrhizic acid and glycyrrhetinic acid in the supernatant and precipitate, and the final...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com