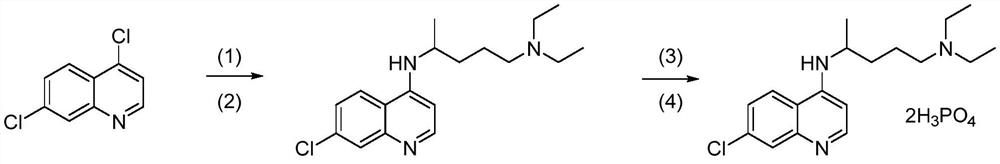
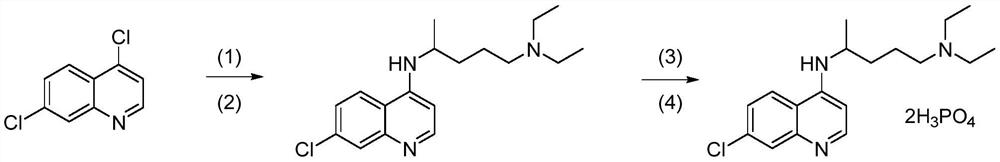
Synthesis method of high-purity chloroquine phosphate
A technology of chloroquine phosphate and a synthesis method, applied in directions such as organic chemistry, can solve problems such as high reaction temperature and uneconomical production, and achieve the effects of lowering reaction temperature, reducing side reactions, and reducing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
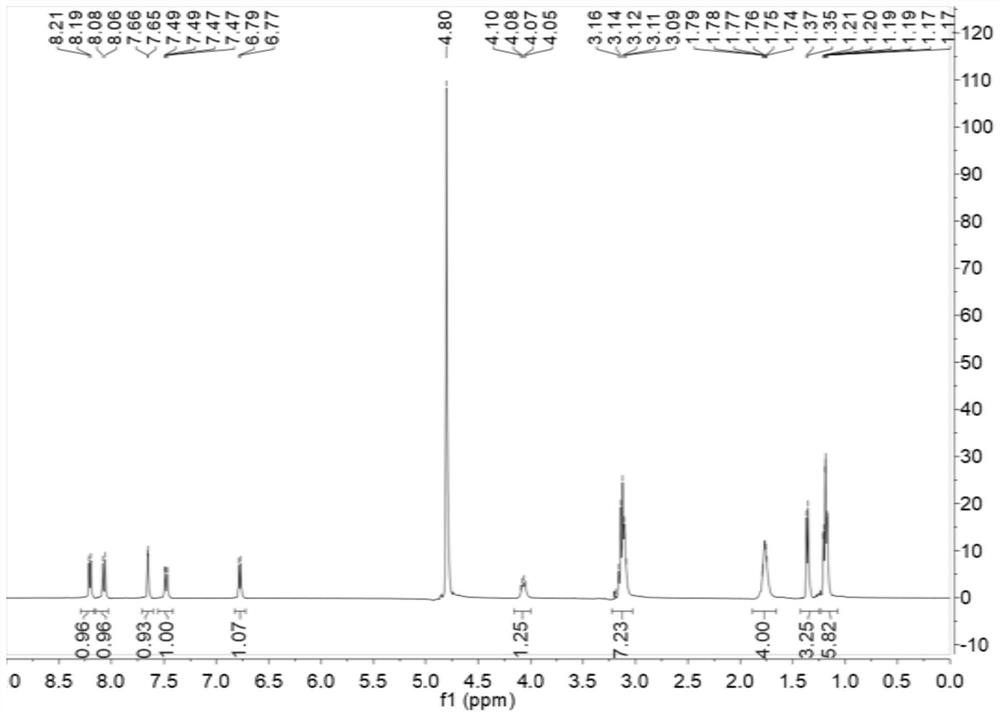
[0044] (1) prepare chloroquine,
[0045] Mix 198g of 4,7-dichloroquinoline (1mol) and 94g (1mol) of phenol evenly, raise the temperature of the system to 70-80°C, and slowly add 332g of 2-amino-5-diethylaminopentyl under the pressure of 1.7atm alkanes (2.1 mol) and 100 g of di-n-butylamine (0.77 mol). After the reaction was complete, add 600 mL of toluene to dilute, add a mass fraction of 10% sodium carbonate solution to adjust the pH of the system to 8-9, separate liquids, wash with water (200 mL*3), and concentrate the organic phase to dryness to obtain 436.2 g of crude chloroquine. Save the aqueous phase for later use.
[0046] (2) Refined Chloroquine
[0047] Add 150ml sherwood oil in 436.2g of chloroquine crude product, heat and dissolve clear, stir and crystallize in ice-water bath, filter, dry to obtain 294.2g chloroquine. Molar yield: 90.55%, liquid chromatography purity (HPLC): 98.42%. The mother liquor of petroleum ether was reserved for future use.
[0048] (3)...
Embodiment 2
[0054] (1) prepare chloroquine,
[0055] Mix 178.2g (0.9mol) of 4,7-dichloroquinoline and 84.6g of phenol (0.9mol) evenly, raise the temperature of the system to 80-90°C, and slowly add 300.6g of 2-amino-5-diethylaminopentane (1.9mol) and 80g of di-n-propylamine (0.80mol), react under the pressure of 1.4atm, add 600mL of dichloromethane to dilute after the reaction is complete, add 10% sodium hydroxide solution to adjust the pH of the system to 8-9, separate liquid, wash with water (Totally 540mL three times), concentrated to dryness to obtain 396.0 grams of chloroquine crude product. Save the aqueous phase for later use.
[0056] (2) Refined Chloroquine
[0057] Add 120ml sherwood oil in 396.0g of chloroquine crude product, heat and dissolve clear, stir and crystallize in ice-water bath, filter, dry to obtain product 265.2g chloroquine. Molar yield: 90.32%, purity: 98.17%. The mother liquor of petroleum ether was reserved for future use.
[0058] (3) prepare chloroquine ...
Embodiment 3
[0063] (1) prepare chloroquine,
[0064] Mix 227.7g of 4,7-dichloroquinoline (1.15mol) and 108.1g of phenol (1.15mol) uniformly, heat up the system to 90-100°C, and slowly add 397g of 2-amino-5-diethylaminopentane ( 2.5mol) and 70g of diethylamine (0.96mol), under 1.5atm pressure, heat preservation reaction. After the reaction was complete, 600 mL of chloroform was added for dilution, and 10% potassium hydroxide solution was added to adjust the pH to 8-9, separated, washed with water (570 mL three times), and concentrated to dryness to obtain 522.9 g of crude chloroquine. Save the aqueous phase for later use.
[0065] (2) Refined Chloroquine
[0066] Add 200ml sherwood oil heating to dissolve clear in 522.9g of chloroquine crude product, stir and crystallize in ice-water bath, filter, dry to obtain product 338.5g chloroquine. Molar yield: 90.72%, purity: 98.55%. The mother liquor of petroleum ether was reserved for future use.
[0067] (3) prepare chloroquine phosphate
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com