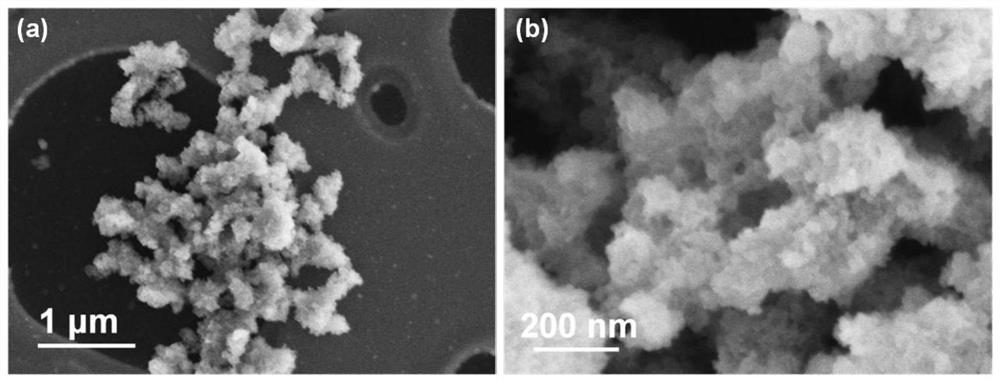
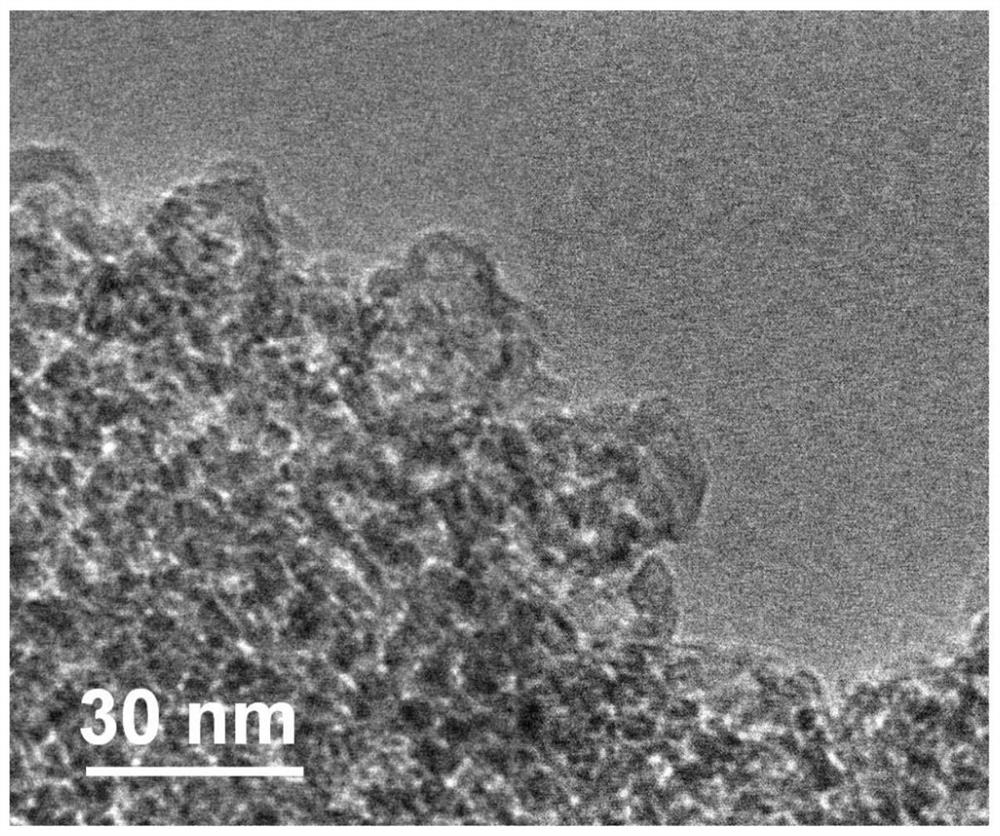
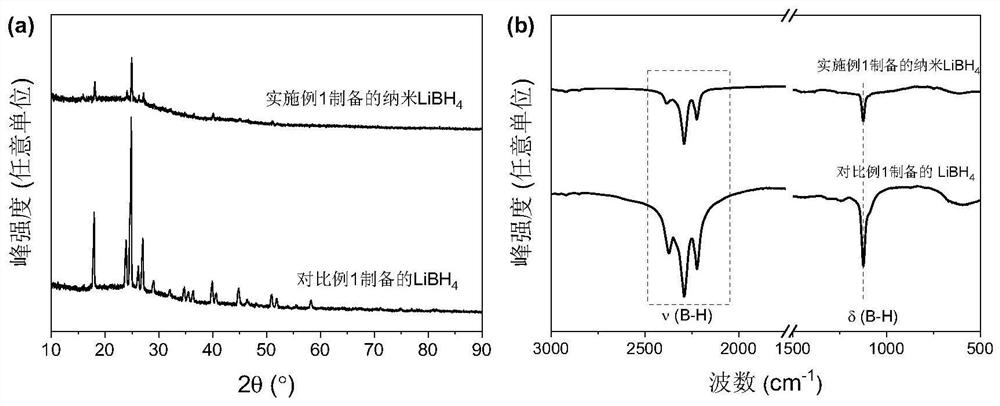
A kind of nano lithium borohydride, its in-situ preparation method and application
An in-situ preparation technology of lithium borohydride, which is applied in the field of hydrogen storage materials and nanomaterials, can solve the problems of low filling efficiency of carrier materials, low nanometerization effect, troublesome operation, etc., and achieve improved hydrogen absorption and desorption kinetics, high Capacity, the effect of inhibiting agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 prepares nano lithium borohydride
[0065] Add 2ml of butyllithium solution (2M / L) and 1ml of triethylamine borane dropwise into 70ml of n-hexane in an argon atmosphere glove box, stir and ultrasonically mix thoroughly, and then put them into a ventilated reactor. Fill the reactor with 50bar high-purity hydrogen, and heat and stir the reactor in an oil bath at 100°C for 24 hours. During the heating process, butyllithium (Li-C 4 h 9 ) hydrogen absorption to become lithium hydride (LiH), followed by triethylamine borane (C 6 h 18 BN) combined to form triethylamine adducts of lithium borohydride (LiBH 4 ·C 6 h 15 N).
[0066] After the reaction was completed, the reactor was moved into the glove box, and the reaction product was subjected to suction filtration, and the solid product (LiBH 4 / C 6 h 15 N), put the product in 500ml of n-hexane, stir and sonicate for 12h to remove the triethylamine adduct. Subsequently, the white powdery product was obta...
Embodiment 2
[0075] In this embodiment, graphene is selected as a carrier, and lithium borohydride nanoparticles are loaded between the graphene layers to realize the loading of nanometer lithium borohydride, so as to reduce the scale of lithium borohydride and obtain better dehydrogenation performance.
[0076] (1) According to the molar ratio of graphene and butyl lithium: 0.2:1, 0.4:1, 0.8:1 and 1.2:1 respectively, take 9.6mg, 19.2mg, 38.4mg and 57.6mg of graphene in argon Add 80ml of n-hexane into the atmosphere glove box, stir and ultrasonic for 2 hours to fully disperse the graphene, expand the graphene layer, which is conducive to the loading of lithium borohydride, and the ultrasonic power is 300W, to obtain graphene / n-hexane mixtures with different graphene contents.
[0077] (2) Add 2ml of butyllithium solution (2M / L) and 1ml of triethylamine borane dropwise into the graphene / n-hexane mixture prepared in step (1) in an argon atmosphere glove box, and stir and ultrasonically mix th...
Embodiment 3
[0091] In this example, graphene is selected as the carrier, nickelocene is selected as the transition metallocene as the catalyst precursor, and lithium borohydride nanoparticles and Ni nanoparticles are loaded between the graphene layers to achieve graphene confinement and nano-Ni catalysis. Synergistic modification for better dehydrogenation performance. The molar ratio of graphene to butyl lithium: 0.8:1, the molar ratio of nickelocene to butyl lithium: 0.05:1
[0092] (1) Get 38mg of graphene and 68mg of nickelocene and add in 80ml of n-hexane in an argon atmosphere glove box, stir and ultrasonic for 2h, the ultrasonic power is 300W, fully disperse graphene, unfold the graphene layer, nickelocene is in dissolved in n-hexane.
[0093] (2) Add 2ml of butyllithium solution (2M / L) and 1ml of triethylamine borane into the mixture of graphene, ferrocene and n-hexane dropwise in an argon atmosphere glove box, and stir and ultrasonically mix thoroughly Put it into a ventilated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com