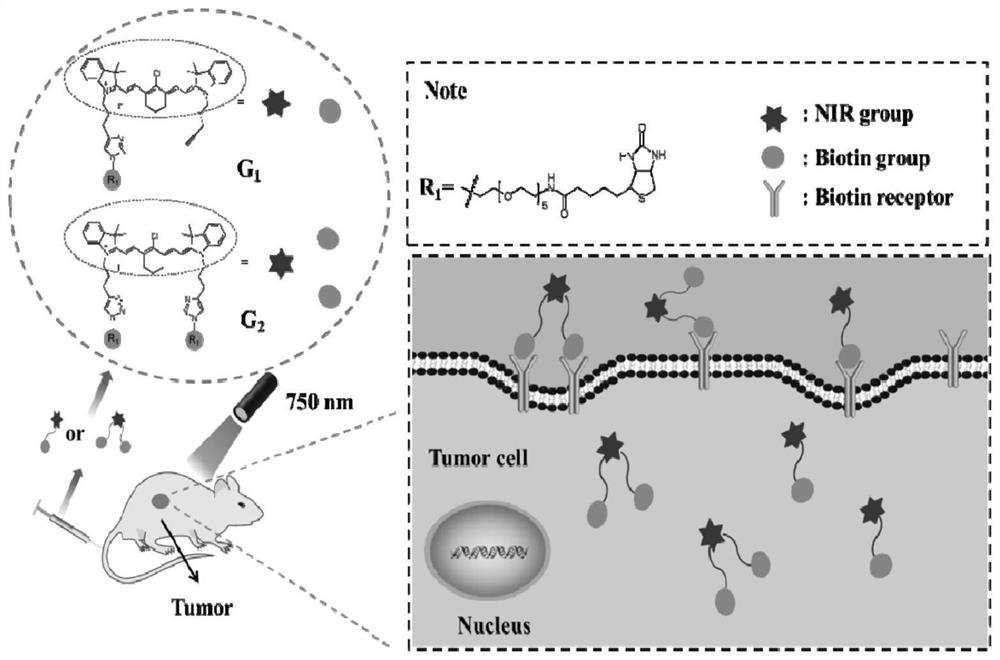
Fluorescent imaging probe and preparation method and application thereof
A fluorescence imaging agent and probe technology, applied in the field of fluorescence imaging, can solve the problems of unpredictable cancer cells, easy transmembrane transport into tumor cells, tumor cell retention time, easy aggregation, etc. The effect of strong membrane transport and long residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
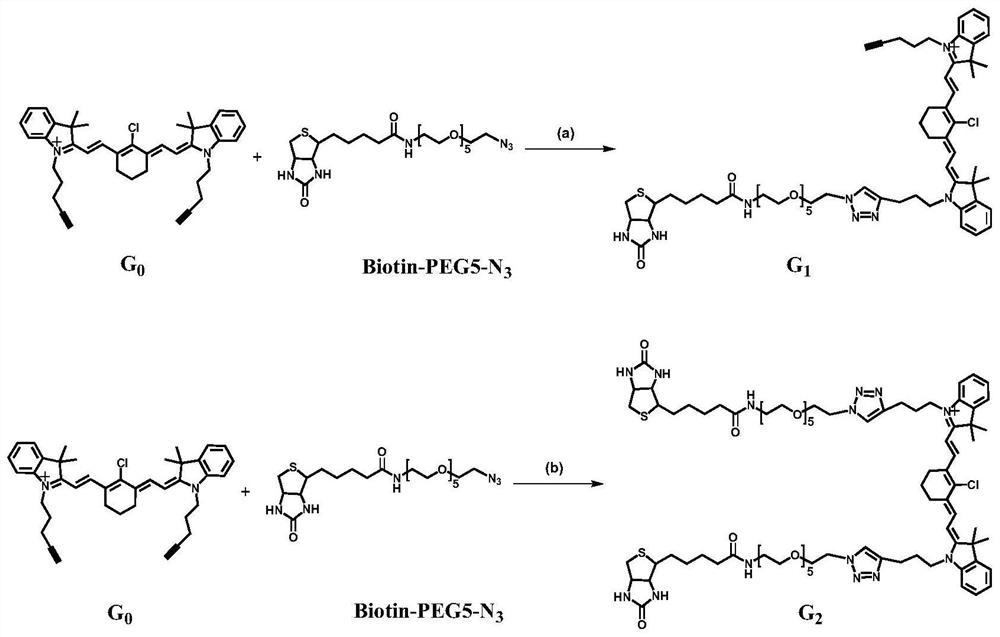
[0063] Example 1 Compound G 1 and G 2 Synthesis
[0064] 1 Compound G 0 Synthesis
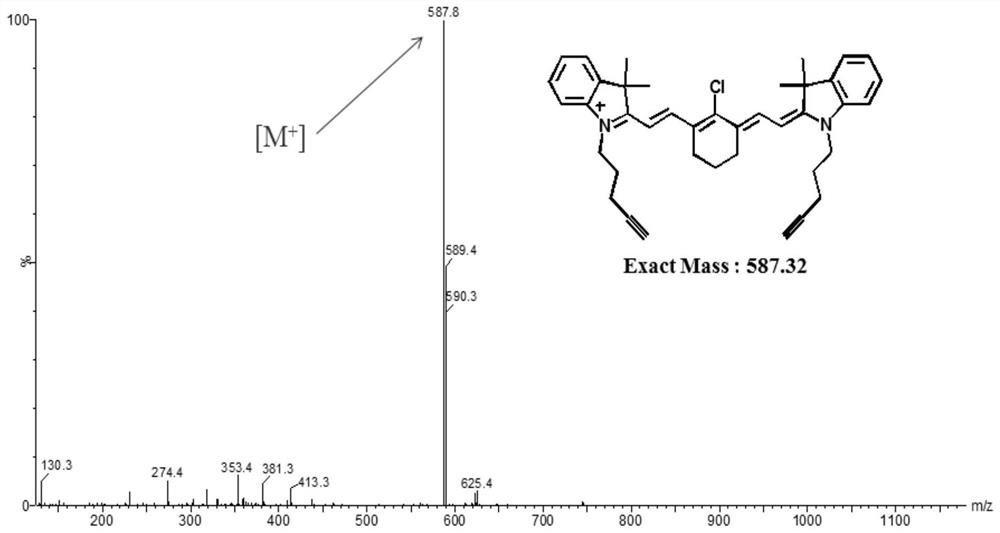
[0065] According to the reported method (Chemical communications2017,53,6117-6120), G 0, specific method: KI (5.8g, 34.93mmol, 6eq) was dissolved in acetonitrile (25mL); then 5-chloro-1-pentyne (1841 μL, 17.45mmol, 3eq) was added. The reaction solution was stirred at 50°C for 40 minutes. To this was added 2,3,3-trimethylindoline (933 μL, 5.82 mmol, 1 eq). The reaction mixture was heated at 85°C for 24 hours. The reaction liquid was filtered, and then acetonitrile was removed by distillation under reduced pressure. The resulting solid was purified by column chromatography (mobile phase: MeOH and DCM volume ratio: 2-5%) to obtain compound 1 in a yield of 17% (224mg, 0.991mmol). Compound 1 (221 mg, 0.977 mmol, 1 eq) and 2-chloro-1-formyl-3-(hydroxymethylene)-1-cyclohexene (84.1 mg, 0.489 mmol, 0.5 eq) were dissolved in anhydrous acetonitrile (3 mL). Acetic anhydride (123 μL) and sodium a...
Embodiment 2
[0078] Determination of photochemical and photophysical properties of embodiment 2 compounds G1 and G2
[0079] 1 Determination of absorption spectrum and fluorescence spectrum
[0080] Methanol was chosen as the solvent to prepare G 0 ,G 1 and G 2 The stock solution (20mM) was used to measure the spectrum. Dilute G with methanol and water, respectively 0 , G 1 and G 2 The stock solutions were diluted to different concentrations (4 μM, 3 μM, 2 μM, 1 μM, 0.5 μM) to obtain absorption spectra. Absorbance fit straight lines at λmax and concentration were obtained. Prepare 4 µM G 0 , G 1 and G2 solution to measure the fluorescence spectrum. The present invention uses methanol and water as solvents respectively to compare the fluorescence intensity of probes in different solvents.
[0081] 2 Determination of photon quantum yield
[0082] Prepare G with methanol and water as solvents, respectively 1 , G 2 and indocyanine green (ICG) solution (1 μM); ICG was used as a re...
Embodiment 3
[0095] Example 3 Tumor cell-specific imaging in vitro
[0096] 1 cell culture
[0097] To verify the specific accumulation of the probe at the tumor site, HeLa cells were selected as BR-positive tumor cells, and LO2 cells were selected as BR-negative normal cells. HeLa and LO2 cells were placed in high glucose DMEM medium containing 10% (v / v) fetal bovine serum (FBS) in 5% (v / v) CO 2 Cultivated in a humid and stable environment. The incubation temperature was 37°C.
[0098] 2 Cytotoxicity assay
[0099] Perform MTT assay to evaluate probe G 1 and G 2 cytotoxicity. HeLa and LO2 cells were seeded into 96-well plates, and the density was controlled at 10,000 cells per well. Cells were incubated at 37°C for 24h to allow cell attachment. Will G 1 and G 2 respectively dissolved in DMF to obtain 20mM G 1 and G 2 Stock solution, G 1 and G 2 The stock solution was diluted to the target concentration. After removing the medium, probe solutions at different concentrations ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com