Nitridation synthesis of nitrogen oxide material SmTiO2N and application of nitrogen oxide material SmTiO2N in field of photocatalysis
A nitrogen oxide, photocatalytic technology, applied in hydrogen/syngas production, physical/chemical process catalyst, chemical/physical process, etc., can solve problems such as nitriding synthesis, and achieve the effect of low temperature and shortened nitriding time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
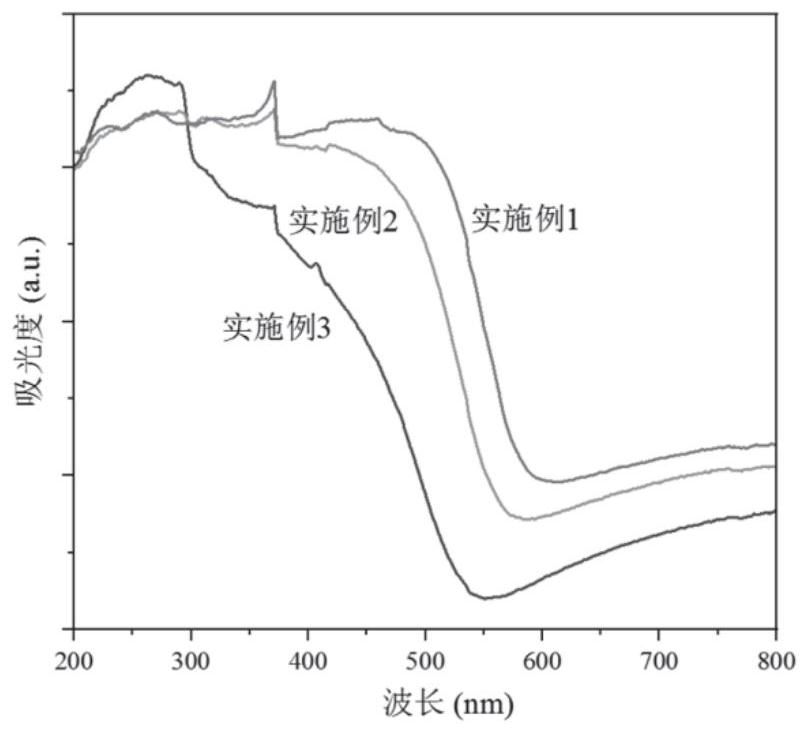
Embodiment 1
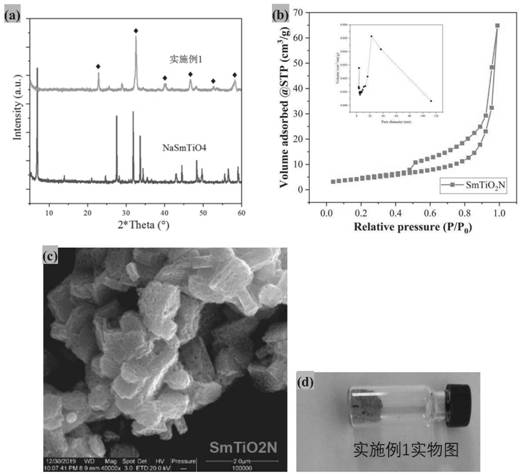
[0048] (1) Synthesis of composite bimetallic sodium salt NaSmTiO by solid phase method 4 (Concrete method reference: Journal of SolidState Chemistry 161,225~232 (2001)), the NaSmTiO that adopts excessive deionized water to wash synthesis 4 Until the washing liquid is neutral, dry the solid sample NaSmTiO 4 Synthesized as SmTiO 2 Precursor of N;
[0049] (2) Take 0.5gNaSmTiO 4 In the quartz hanging basket, place the quartz hanging basket on the air flow baffle in the center of the quartz tube, seal the quartz tube and connect the NH 3 piping;
[0050] (3) Turn on NH 3 , the flow rate is 100ml / min, purging at room temperature for 30min, then heating up to 950℃ at 2℃ / min, nitriding at a constant temperature of 950℃ for 6h, and taking it out after cooling down to room temperature naturally after nitriding;
[0051] (4) Disperse the obtained powder in 20 mL of nitric acid with a concentration of 1 mol / L, stir for 30 minutes, centrifuge the powder sample and wash it with water...
Embodiment 2
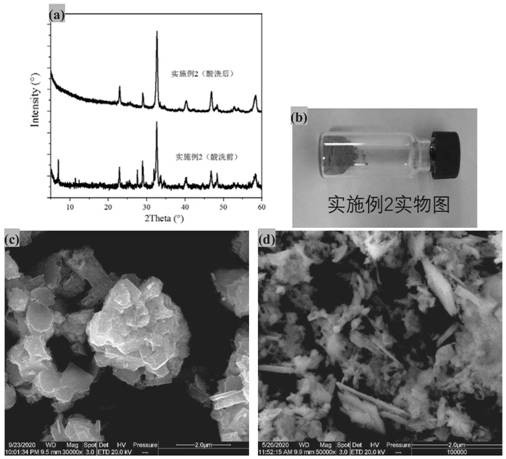
[0053] (1) Synthesis of composite bimetallic sodium salt NaSmTiO by solid phase method 4 (Concrete method reference: Journal of SolidState Chemistry 161,225~232 (2001)), the NaSmTiO that adopts excessive deionized water to wash synthesis 4 Until the washing liquid is neutral, dry the solid sample NaSmTiO 4 Synthesized as SmTiO 2 Precursor of N;
[0054] (2) Take 0.5g NaSmTiO 4 In the corundum porcelain boat, place the corundum porcelain boat in the center of the horizontal tube furnace, seal and connect the NH 3 piping;
[0055] (3) Turn on NH 3 , the flow rate is 200ml / min, after purging at room temperature, the temperature is raised to 850°C at 5°C / min, and the constant temperature is 850°C for nitriding for 8 hours. After the nitriding is completed, the temperature is naturally lowered. Take out the powder after reaching room temperature;
[0056] (4) Disperse the obtained powder in 20 mL of aqua regia (concentrated hydrochloric acid mixed with concentrated nitric ac...
Embodiment 3
[0058] (1) Synthesis of composite bimetallic sodium salt NaSmTiO by solid phase method 4 (Concrete method reference: Journal of SolidState Chemistry 161,225~232 (2001)), the NaSmTiO that adopts excessive deionized water to wash synthesis 4 Until the washing liquid is neutral, dry the solid sample NaSmTiO 4 Synthesized as SmTiO 2 Precursor of N;
[0059] (2) Take 0.5gNaSmTiO 4 In the quartz hanging basket, place the quartz hanging basket on the air flow baffle in the center of the quartz tube, seal the quartz tube and connect the NH 3 piping;
[0060] (3) Turn on NH 3 , the flow rate is 400ml / min, purging at room temperature for 30min, then heating up to 800℃ at 1℃ / min, constant temperature nitriding for 20h, after nitriding, naturally cool down to room temperature and take out;
[0061] (4) Disperse the obtained powder in 20 mL of aqua regia, stir for 2 hours, centrifuge the powder sample and wash it with water, and then dry it to obtain the pure phase of SmTiO 2 N powd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com