Preparation method of roxadustat intermediate IV
A technology for roxadustat and intermediates, which is applied in the field of preparation of roxadustat intermediate IV, can solve the problems of unsuitability for large-scale production, unfavorable industrial amplification, and high equipment requirements, and achieve improved safety factor, low cost, The effect of simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
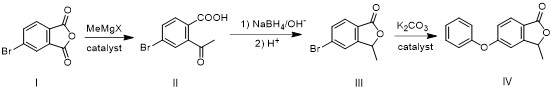
[0052] This embodiment is a preparation method of roxadustat intermediate IV, and its preparation includes the following steps:
[0053] Step 1. Intermediate II is obtained by addition of 4-bromophthalic anhydride (compound I) and methyl Grignard reagent: Step 2. Intermediate II is first reduced by sodium borohydride, and then cyclized under acidic conditions to form Intermediate III: Step 3, intermediate III and phenol undergo aryl alkylation reaction to obtain roxadustat intermediate IV, such as figure 1 .
[0054] In addition: the present invention also proposes following subsidiary technical scheme:
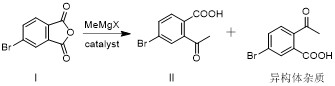
[0055] In the preparation process of intermediate II, the methyl Grignard reagent used in the anhydride ring-opening reaction process is methylmagnesium bromide or methylmagnesium chloride, preferably methylmagnesium bromide;
[0056] In the preparation process of intermediate II, the reaction temperature is controlled at -10~5°C during the ring-opening reaction of the acid ...
Embodiment 1
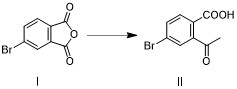
[0064] Example 1: Preparation of intermediate II (4-bromo-2-acetylbenzoic acid), such as image 3 .
[0065] Add 4-bromophthalic anhydride I (45.4g, 200mmol) and tetrahydrofuran (180mL), 0.1eq. cuprous iodide to the three-necked flask, stir to dissolve, cool to -10~0°C and switch nitrogen gas 3 times under vacuum , under the protection of nitrogen, slowly drop methylmagnesium chloride tetrahydrofuran solution (2.0M, 110mL) into the reaction bottle, keep it warm for 2-4h after the drop, after the reaction, add 1mol / L dilute hydrochloric acid (440mL) to quench the reaction , the aqueous phase was extracted twice with ethyl acetate (180mL), the combined organic phase was washed once with saturated brine (180mL), dried over anhydrous sodium sulfate, concentrated and recrystallized with a mixed solvent of petroleum ether ethyl acetate to obtain intermediate II ( 39.5g, 81.27%).
[0066] MS m / z 244 [M+H]+ 1H-NMR (400Hz, CDCl3-d6) δ1.90 (s, 3H), 7.70-7.77 (m, 3H).
Embodiment 2
[0067] Example 2: Preparation of intermediate III [3-methyl-5-bromoisobenzofuran-1(3H)-one], such as Figure 4 .
[0068] Add 5% sodium hydroxide solution (120g) and 4-bromo-2-acetylbenzoic acid II (36.45g, 150mmol) into the three-necked flask, stir at room temperature for 1h, cool down to 0~10°C, add hydroboration in batches Sodium (6.8g), after the addition was complete, stirring was continued for 3h. After the reaction is complete, add dichloromethane, adjust the pH to 2 with 6mol / L hydrochloric acid, stir well, let it stand for layering, separate liquids, extract the aqueous phase twice with dichloromethane, combine the organic phases, and wash with 5% carbonic acid Wash with sodium hydrogen solution until neutral, wash once with purified water, and dry over anhydrous magnesium sulfate. Filtration and distillation under reduced pressure gave an oily liquid, which was recrystallized from ethyl acetate to give Intermediate III (25.88g, 76.04%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com