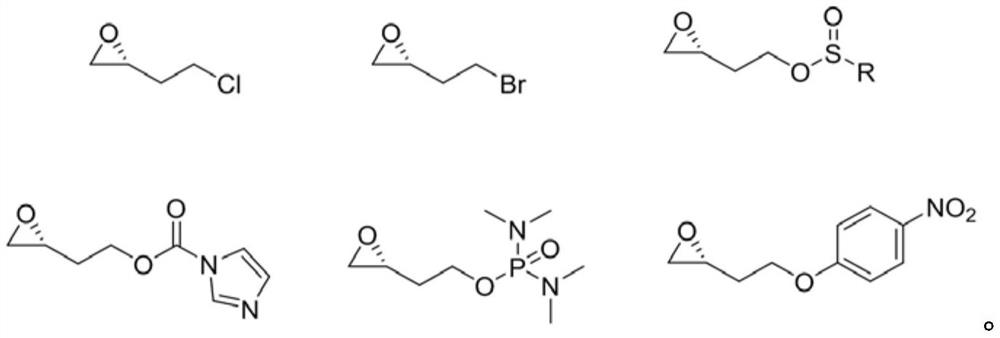
Preparation method of vernakalant hydrochloride
A technology of venakaran hydrochloride and solvent, which is applied in the field of chemical synthesis, can solve the problems of long synthesis route, heavy metal residue, difficulty in industrial scale up, etc., and achieves the effect of facilitating development and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 (R)-1-((1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexylpyrrol-3-ol hydrochloride (Venakalant hydrochloride ) preparation
[0038] In a single-necked bottle, add (1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexylamine (14.0g, 50mmol), 200ml of acetonitrile, electromagnetically stir until completely dissolved, add anhydrous Potassium carbonate (8.3g, 60mmol), add R-1,2 epoxybromobutane (7.6g, 50mmol) dropwise, heat up to reflux, control the system temperature at 70-80°C, and keep the reaction time for 6 hours, filter, and filter out inorganic Salt, add 5M hydrochloric acid / methanol solution (100ml) to the organic phase and stir for 1 hour until the salt is completely formed. The organic phase is evaporated to remove the solvent under reduced pressure to obtain the crude product of vernakalant as a light-colored viscous substance, which is separated by adding isopropyl ether and beating , and filtered to obtain 13.41 g of (R)-1-((1R,2R)-2-(3,4-dimethoxyphenethoxy)cycloh...
Embodiment 2
[0040] Example 2 (R)-1-((1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexylpyrrol-3-ol hydrochloride (Venakalant hydrochloride ) preparation
[0041] In a single-necked bottle, add (1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexylamine (14.0g, 50mmol), 200ml of acetonitrile, electromagnetically stir until completely dissolved, add anhydrous Potassium carbonate (8.3g, 60mmol), add R-1,2 epichlorobutane (5.3g, 50mmol) dropwise, heat up to reflux, control the system temperature at 70-80°C, reaction time 6h, filter, filter out inorganic salts , add 5M hydrochloric acid / methanol solution (100ml) to the organic phase and stir for 1h until the salt is completely formed. The organic phase is evaporated to remove the solvent under reduced pressure to obtain the crude product of vernakalant as a light-colored viscous substance, which is recrystallized by adding isopropyl acetate. , and filtered to obtain 14.41 g of (R)-1-((1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexylpyrrol-3-ol hydrochloride as ...
Embodiment 3
[0043]Example 3 (R)-1-((1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexylpyrrol-3-ol hydrochloride (Venakalant hydrochloride ) preparation
[0044] In a single-necked bottle, add (1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexylamine (14.0g, 50mmol), 200ml tetrahydrofuran, stir until completely dissolved, add DBU ( 9.1g, 60mmol), add (R)-4-methylbenzenesulfonyloxy-1,2-epoxybutane (12.1g, 50mmol) dropwise, slowly raise the temperature to reflux, control the system temperature at 60-70°C, The reaction time is 6h, after the reaction is complete, add 200ml of purified water, extract 2 times with ethyl acetate 200ml, add 5M hydrochloric acid / methanol solution (100ml) to the organic phase and stir for 1h until the salt is completely formed, evaporate the solvent under reduced pressure to obtain Wiener The crude product of Kalan is a light-colored viscous substance, which is recrystallized by adding isopropyl acetate, and filtered to obtain (R)-1-((1R,2R)-2-(3,4-dimethoxyphenethoxy Base) cy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com