Electrode, electrolyte thin layer and preparation method thereof
A technology of electrolyte and solid electrolyte, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, battery electrodes, etc., can solve problems such as increased production costs, chemical instability of sulfides, and narrow electrochemical stability window of sulfide electrolytes, and achieves improved Effects of energy density, increased energy density, and good solid-solid contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
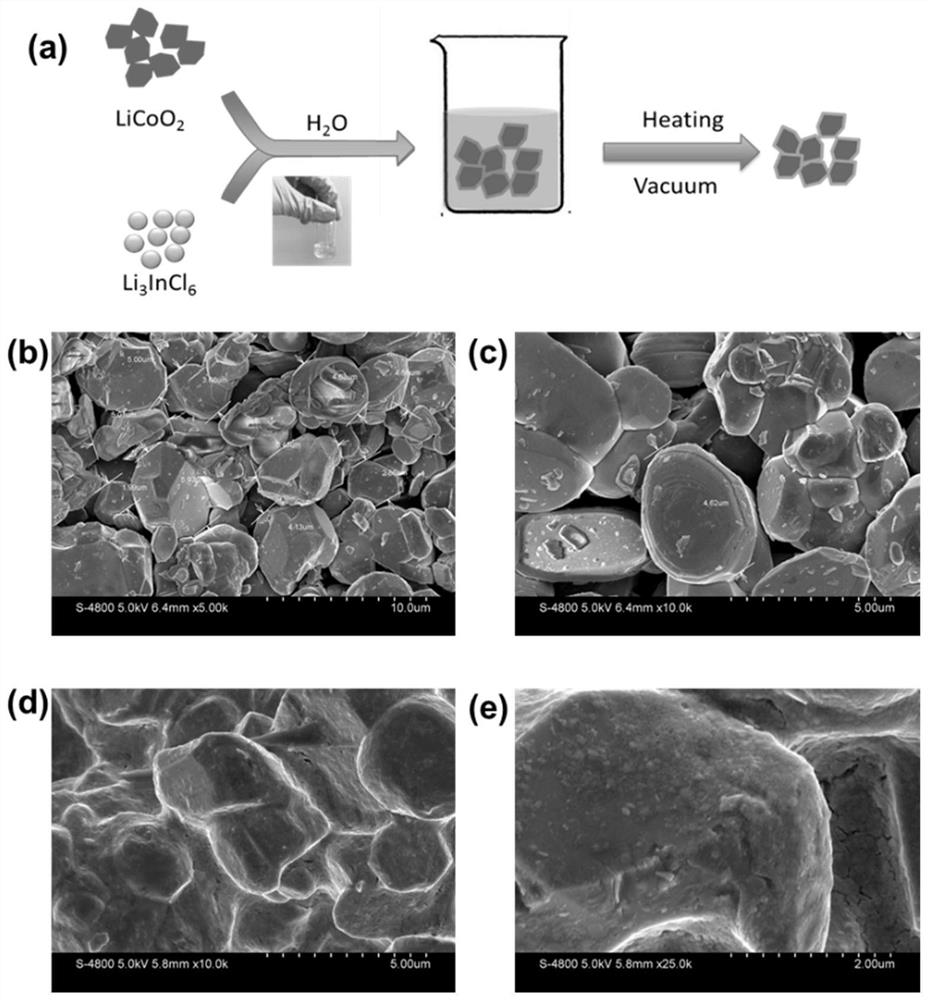
[0090] Embodiment 1 aqueous phase forms Li 3 InCl 6 Coated LiCoO 2 Cathode material
[0091] 75mg of Li 3 InCl 6 Dissolve in 2g of water, then add 425mg of LiCoO 2 , dried at 100°C, and then transferred to a vacuum oven at 200°C for further dehydration and drying to obtain L i3 InCl 6 Coated LiCoO 2 ; The whole experimental process does not need inert atmosphere protection.
[0092] figure 1 Among them, (a) represents the specific synthesis process; Heating represents heating, Vacuum represents the vacuum condition; (b,c) represents the LiCoO before coating 2 SEM photos of ; (d, e) represent the coated LiCoO 2 SEM photographs.
Embodiment 2
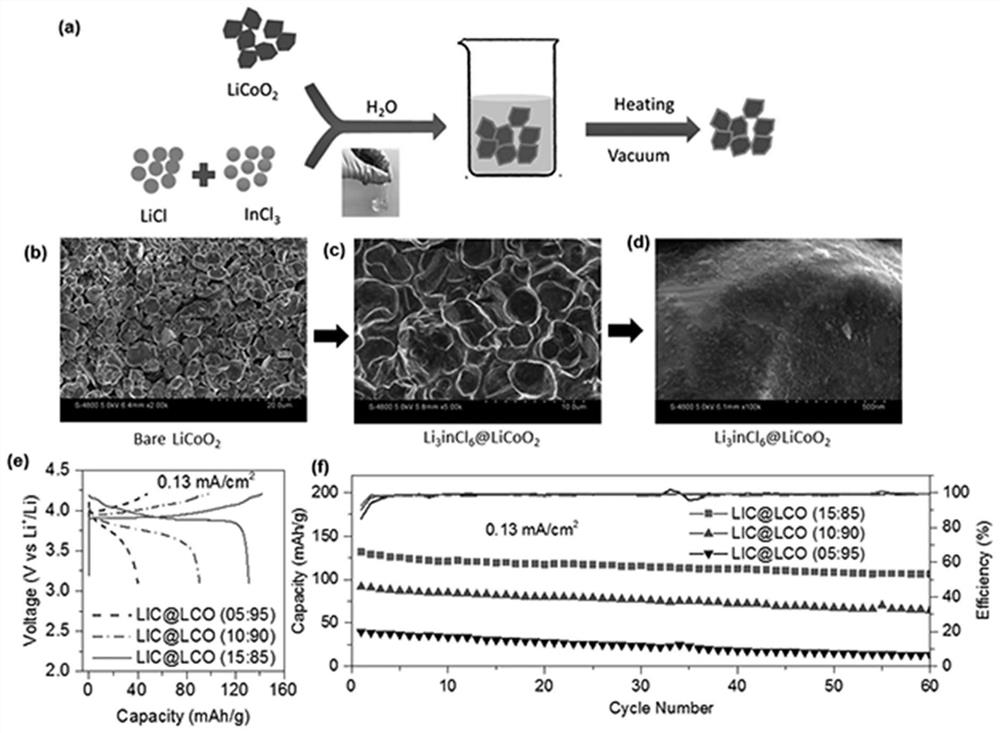
[0093] Embodiment 2 aqueous phase forms Li in situ 3 InCl 6 Coated LiCoO 2 Cathode material
[0094] 27.4mg of LiCl and 47.6mg of InCl 3 Dissolve in water, then add 425mg of LiCoO 2 , placed in a 100-degree oven to evaporate to dryness, and then transferred to a 200-degree vacuum oven to react for 5 hours. get Li 3 InCl 6 Coated LiCoO 2 , (Li 3 InCl 6 with LiCoO 2 The mass ratio is 15:85). The whole experimental process does not need inert atmosphere protection.
[0095] figure 2 Among them, (a) indicates the specific synthesis process; Heating indicates heating, and Vacuum indicates vacuum conditions; (b) LiCoO before coating 2 SEM images of ; (c,d) coated LiCoO 2 SEM photos of ; (e) coated with different contents of Li 3 InCl 6 LiCoO 2 The first charge-discharge curve; (f) Coating with different contents of Li 3 InCl 6 LiCoO 2 cycle stability. The abscissa of (e) represents the discharge specific capacity, and the ordinate represents the voltage to the ...
Embodiment 3
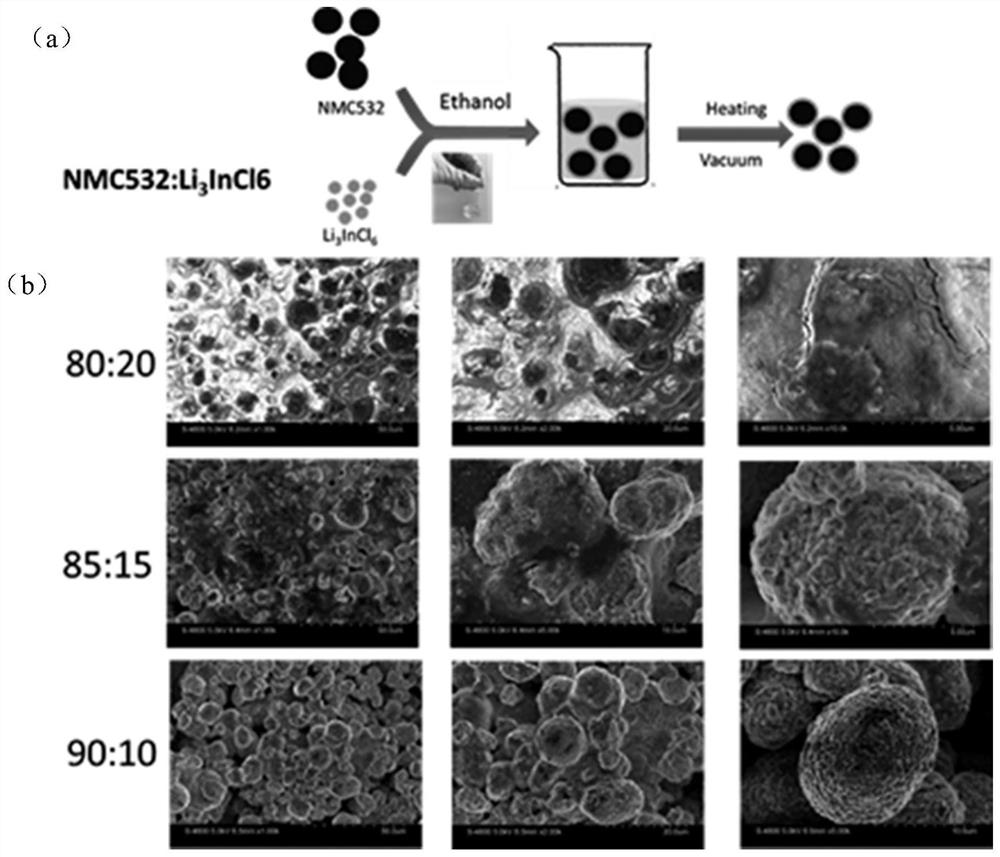
[0096] Embodiment 3 organic phase forms Li 3 InCl 6 Coated NMC532 cathode material
[0097] 75mg of Li 3 InCl 6 and 425 mg of NMC532 in 2 g of ethanol, ultrasonically dispersed for 5 minutes, then transferred to a 100-degree oven for drying, and then transferred to a 200-degree vacuum oven for further desolvation and drying. get Li 3 InCl 6 Coated NMC532 (NMC532 and Li 3 InCl 6 The mass ratio is 85:15), and the whole experimental process does not need inert atmosphere protection.
[0098] Control the NMC532 and Li respectively in basically the same way 3 InCl 6 The mass ratio is 80:20, 90:10, prepared and coated with different contents of Li 3 InCl 6 The NMC532.
[0099] image 3 Among them, (a) represents the specific synthesis process; Ethanol represents ethanol, Heating represents heating, Vacuum represents vacuum conditions; (b) represents the coating with different contents of Li 3 InCl 6 Electron micrograph of NMC532.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com