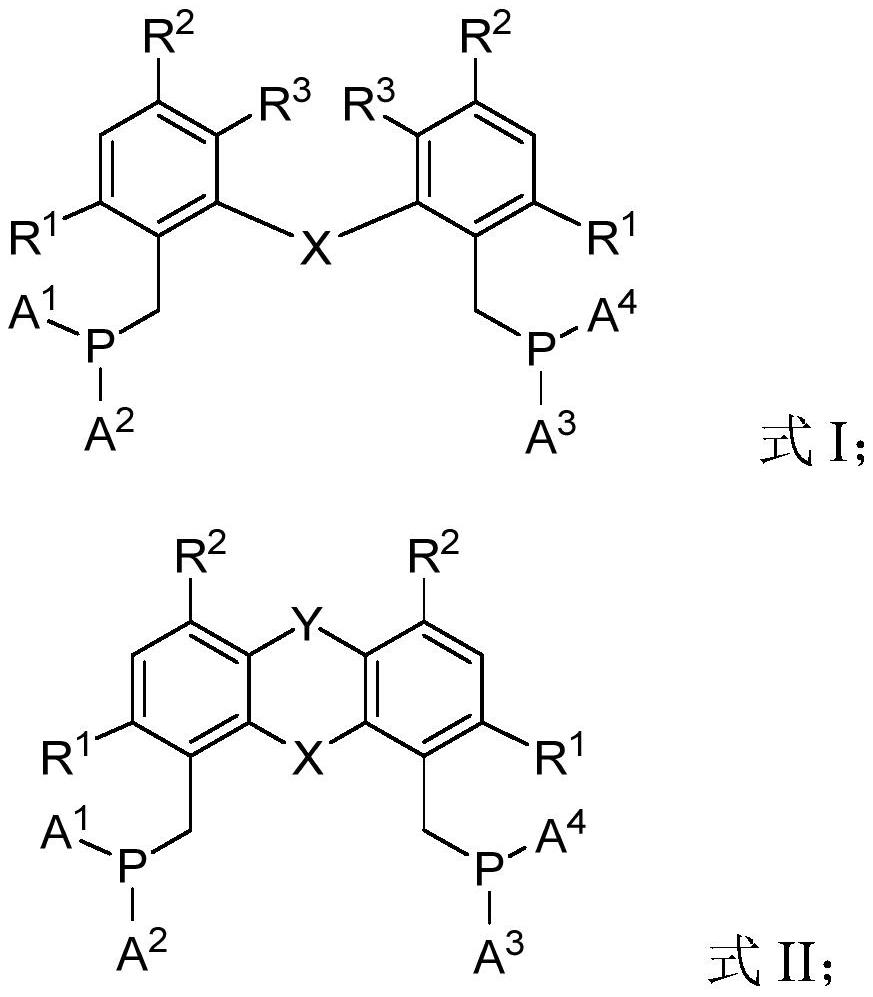
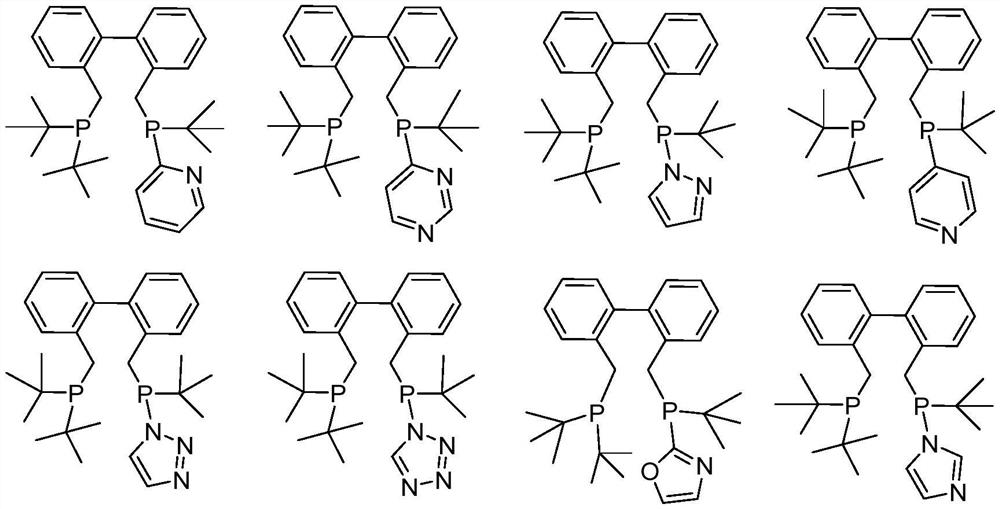
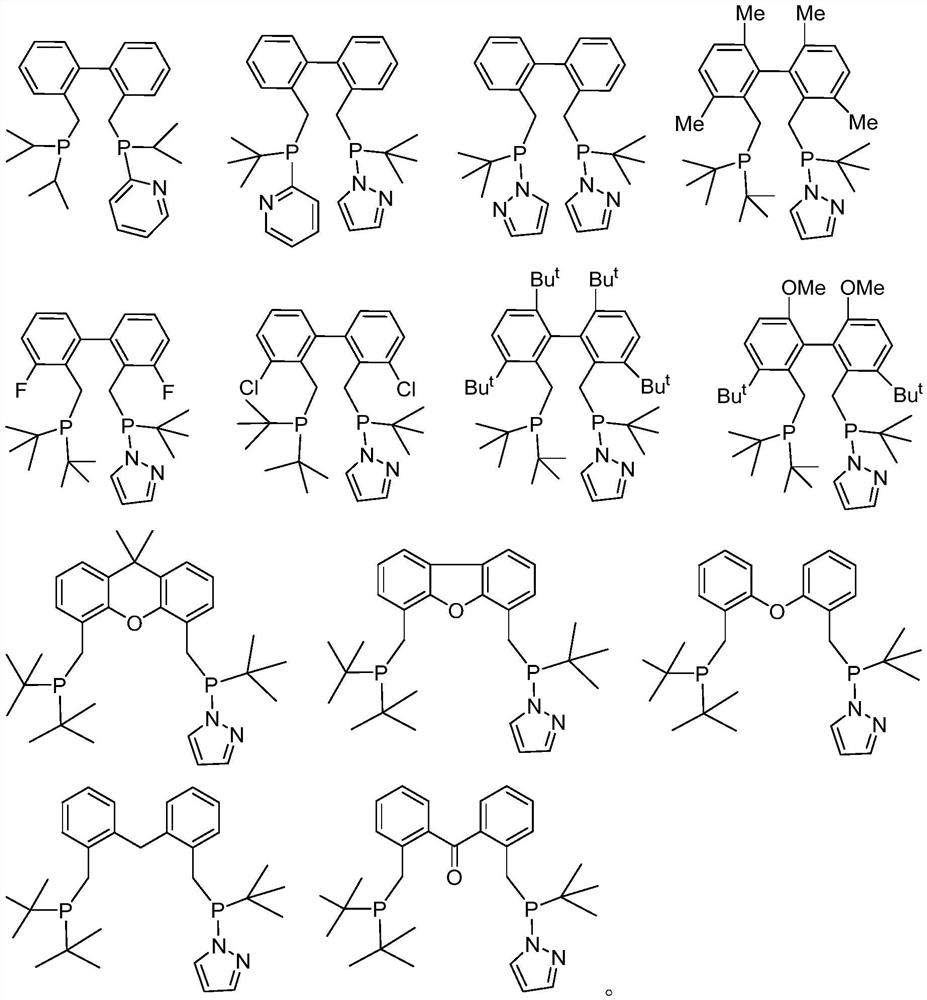
Diphosphine compound, catalyst system containing diphosphine compound and application of diphosphine compound
A phosphine compound and catalyst technology, applied in the field of catalysis, can solve the problems of difficult to meet industrialization conditions, cumbersome steps, unsatisfactory conversion rate and regioselectivity, etc., and achieve the effect of increasing the ability of π feedback electrons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0072] 2-pyridyl tert-butylphosphine chloride The preparation method is as follows:
[0073]
[0074] Add isopropylmagnesium chloride lithium chloride (1.3M, 110mmol) tetrahydrofuran solution to a dry round bottom flask under nitrogen protection, cool to 0°C and add 2-bromopyridine (15.8g, 100mmol) dropwise, drop After the addition was completed, the reaction was continued at 0°C for 2 hours, and then the reaction was continued at room temperature for 2 hours.
[0075] Take another dry round-bottomed flask, add tert-butylphosphorus dichloride (105mmol) and dry tetrahydrofuran (200mL), cool to -40°C, add the above reaction solution dropwise, and continue to react for 12 hours. Warm to room temperature and continue the reaction for another 12 hours. After the reaction was completed, the tetrahydrofuran solution was first distilled off, and then distilled under a reduced pressure of 10 mbar at 125° C. to obtain 15.3 g (76%) of light yellow 2-pyridyl tert-butylphosphine chlo...
preparation example 2
[0077] 1H-pyrazolyl tert-butylphosphine chloride The preparation method is as follows:
[0078]
[0079] Add tert-butylphosphorus dichloride (105mmol), pyridine (120mmol) and dry tetrahydrofuran (200mL) into a dry round-bottomed flask under nitrogen protection, stir well and cool to -20°C, then add pyrazole dropwise (6.8g, 100mmol), after the dropwise addition, continue to react at -20°C for 12 hours, then rise to room temperature and continue to react for 24 hours. After the reaction is completed, first evaporate the dichloromethane solution, then reduce the pressure to 10mbar, and distill at 110°C 15.8 g (83%) of pale yellow 1H-pyrazolyl tert-butylphosphine chloride are obtained.
[0080] The compounds of the synthetic methods not mentioned in the present invention are all raw material products obtained through commercial channels, solvents and reagents used in the present invention, such as methyl alcohol, benzenesulfonic acid, xylene, butadiene, etc., can be obtained fr...
Embodiment 1
[0083] This example provides a bisphosphine compound, specifically 2-(tert-butyl((2'-((di-tert-butylphosphino)methylene)-(1,1'-biphenyl)-2- Substituted) methylene) phosphine) pyridine, the structural formula is as follows:
[0084]
[0085] The specific preparation method is as follows:
[0086] Add 2,2'-bis(magnesium bromide methylene)-1,1'-biphenyl (556mL, 50mmol, 0.09M) into a dry round bottom flask under the protection of nitrogen, cool down to 10°C, drop by drop Di-tert-butylphosphine chloride (9.5 mL, 50 mmol) was added and stirred evenly. After the dropwise addition was completed, the mixture was raised to room temperature and continued to react for 24 hours. After the reaction was completed, 2-pyridyl tert-butylphosphine chloride (10.1 g, 50 mmol) was continuously added, and the reaction was continued at room temperature for 24 hours. After the reaction was completed, it was quenched with saturated brine, and then extracted 3 times with dichloromethane. After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com