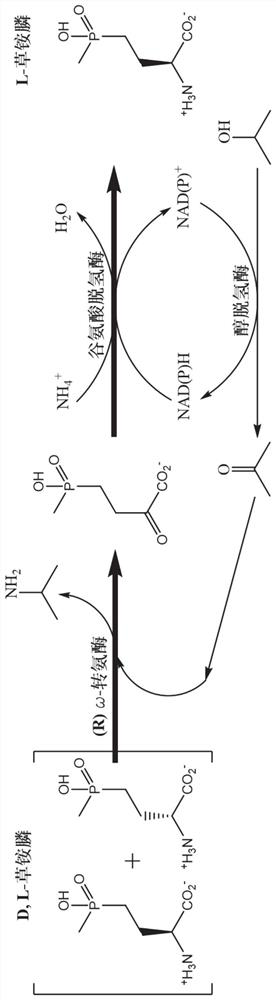
Application of omega-transaminase and method for preparing L-glufosinate-ammonium through deracemization by biological enzyme process
A transaminase, glufosinate-ammonium technology, applied in the biological field, can solve the problems of increased process cost, inactivation of enzyme protein, lack of industrialization potential, etc., and achieves the effect of improving utilization rate and low overall process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The construction of embodiment 1 genetically engineered bacteria
[0043] 1. Construction and screening of ω-transaminase library
[0044] Through literature research, a known R-ω-transaminase was selected, and its gene sequence was obtained from NCBI; the above gene sequence was codon-optimized and sent to Qingke Bioengineering Co., Ltd. for whole gene synthesis, and recombined into pET- 28a(+) plasmid; after correct sequencing, it was introduced into the expression host Escherichia coli E.coil BL21(DE3), and recombinant bacteria were constructed for the subsequent expression of ω-transaminase.
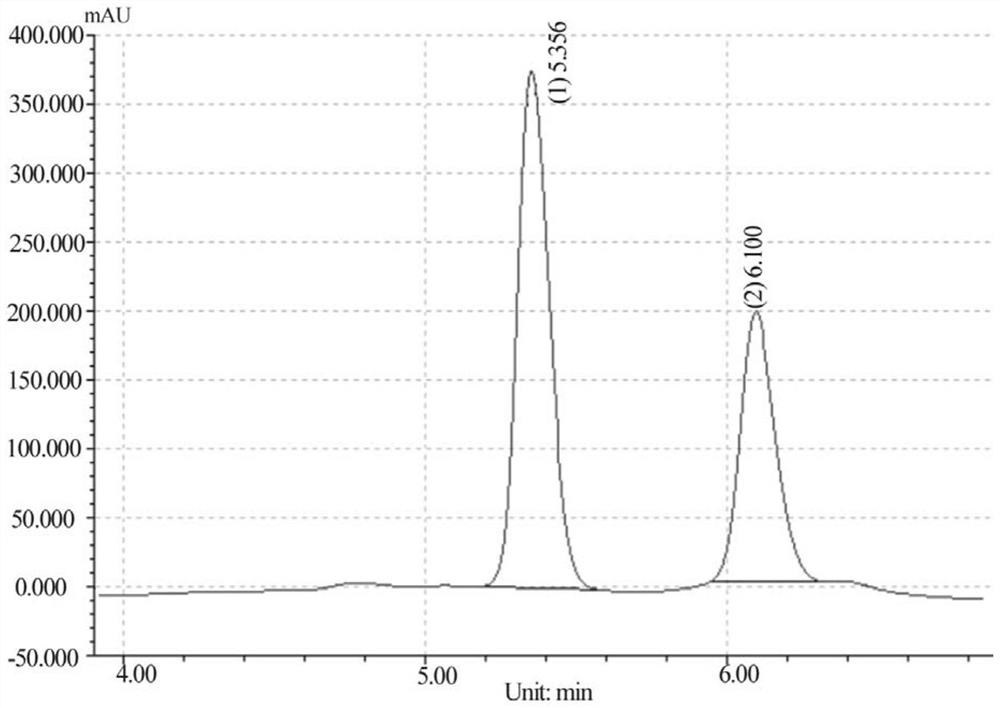
[0045] With D, L-glufosinate-ammonium as the substrate, enzymes with catalytic activity to D-glufosinate-ammonium were screened by HPLC high performance liquid chromatography, and the results are shown in Table 1.
[0046] Table 1 Screening of ω-transaminase
[0047] Numbering source NCBI accession number Enzyme activity U / L TA1 Aspergillus terreus NIH...
Embodiment 2
[0095] 1. The cultivation of microorganisms
[0096] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0097] Genetically engineered bacteria E.coli BL21 (DE3) were inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 500mL fresh LB liquid medium also containing 50μg / mL Kan, shake culture at 37°C until OD 600 When it reaches about 0.8, add IPTG until its concentration is 0.1-0.3mM, and induce culture at 18-28°C for about 20h. After the cultivation, the culture solution was centrifuged at 10,000 rpm for 10 min, the supernatant was discarded, and the bacterial cells were collected, and stored in a -70°C ultra-low temperature refrigerator until use.
[0098] 2. Preparation of crude enzyme solution
[0099] The bacterial cells collected after the cultivation were washed wit...
Embodiment 3
[0106] Select the ω-transaminase derived from Bacillus.Sp YM-01 obtained in Example 1, the glutamic acid dehydrogenase derived from Pseudomonasputida KT2440, and the alcohol dehydrogenase derived from Clostridium beijerinckii, and obtain the crude enzyme liquid after culturing and breaking cells. The enzyme activities were 4.2U / L, 116.9U / L, and 1126.3U / L, respectively.
[0107] Use 0.1M disodium hydrogen phosphate / sodium dihydrogen phosphate buffer (pH 8.0) to prepare 100mM D, L-glufosinate-ammonium, 250mM isopropanol, 500mM ammonium sulfate, 20mM NADP + , 10mM PLP respectively take 2mL, 2mL, 1mL, 1mL, 2mL of the above solution, then add ω-transaminase 1mL, glutamate dehydrogenase and alcohol dehydrogenase 500μL each. The reaction flask was placed in a constant temperature water bath at 40° C. for shaking reaction, and the concentrations of L-glufosinate-ammonium and D-glufosinate-ammonium were detected by HPLC.
[0108] After 72 hours of reaction, the data are as follows: th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pre-denatured | aaaaa | aaaaa |
| Extend | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com