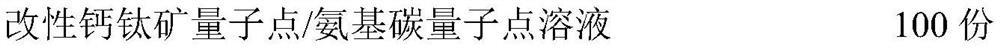Bone defect repair material based on modified perovskite quantum dots/amino carbon quantum dots and preparation method thereof
An amino-carbon quantum and repair material technology, which is applied in the field of bone defect repair materials and its preparation, can solve the problems of aggravating halogen vacancies and interstitial metals, poor stability of perovskite quantum dots, and accelerating the collapse of perovskite structures. Oxidation, excellent metal chelating properties, effects to improve stability and biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
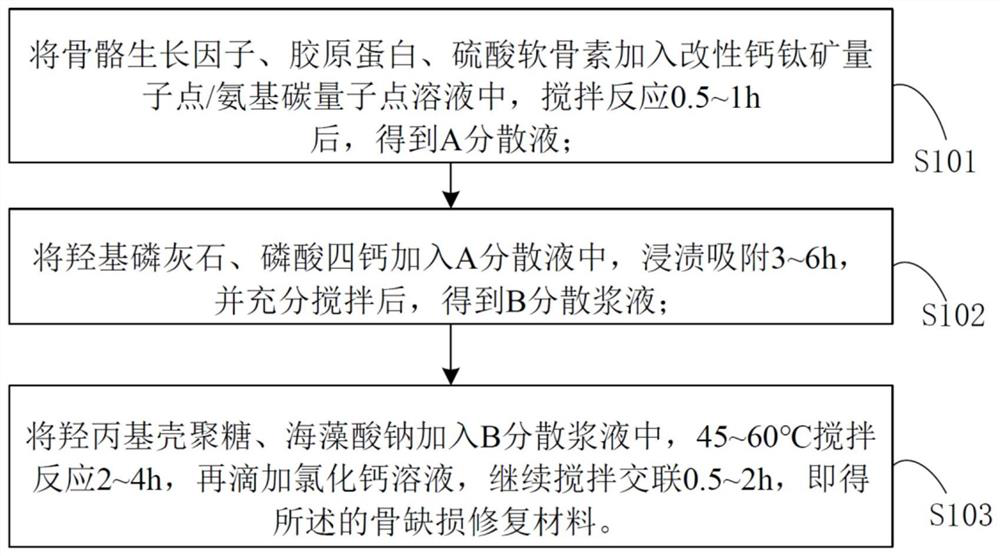
[0031] A bone defect repair material based on modified perovskite quantum dots / aminocarbon quantum dots, comprising the following raw materials in parts by mass:
[0032]
[0033] The raw materials and mass parts of the modified perovskite quantum dot / aminocarbon quantum dot solution are as follows: CsSnCl 3 0.5 parts of perovskite quantum dots, 0.6 parts of amino carbon quantum dots, 8 parts of phosphatidylserine, 3 parts of citric acid, 1 part of reduced glutathione, 0.5 parts of EDC·HCl, 20 parts of chloroform, 100 parts of deionized water .
[0034] A method for preparing a bone defect repair material based on modified perovskite quantum dots / amino carbon quantum dots, comprising the following steps:
[0035] S101: Prepare modified perovskite quantum dot solution:
[0036] i: Add phosphatidylserine to chloroform solvent, magnetically stir until completely dissolved, then add CsSnCl 3 For perovskite quantum dots, after stirring evenly, chloroform was removed by rotary...
Embodiment 2
[0043] A bone defect repair material based on modified perovskite quantum dots / aminocarbon quantum dots, comprising the following raw materials in parts by mass:
[0044]
[0045] The raw materials and mass parts of the modified perovskite quantum dot / aminocarbon quantum dot solution are as follows: CsSnBr 3 0.8 parts of perovskite quantum dots, 1.0 parts of amino carbon quantum dots, 12 parts of phosphatidylserine, 4 parts of citric acid, 2 parts of reduced glutathione, 1 part of EDC·HCl, 25 parts of chloroform, 100 parts of deionized water .
[0046] A method for preparing a bone defect repair material based on modified perovskite quantum dots / amino carbon quantum dots, comprising the following steps:
[0047] S101: Prepare modified perovskite quantum dot solution:
[0048] i: Add phosphatidylserine to chloroform solvent, magnetically stir until completely dissolved, then add CsSnBr 3 For perovskite quantum dots, after stirring evenly, chloroform was removed by rotary ...
Embodiment 3
[0055] A bone defect repair material based on modified perovskite quantum dots / aminocarbon quantum dots, comprising the following raw materials in parts by mass:
[0056]
[0057] The raw materials and mass parts of the modified perovskite quantum dot / aminocarbon quantum dot solution are as follows: CsSnI 3 1 part of perovskite quantum dots, 1.2 parts of aminocarbon quantum dots, 16 parts of phosphatidylserine, 5 parts of citric acid, 3 parts of reduced glutathione, 1 part of EDC·HCl, 30 parts of chloroform, and 100 parts of deionized water.
[0058] A method for preparing a bone defect repair material based on modified perovskite quantum dots / amino carbon quantum dots, comprising the following steps:
[0059] S101: Prepare modified perovskite quantum dot solution:
[0060] i: Add phosphatidylserine to chloroform solvent, magnetically stir until completely dissolved, then add CsSnI 3 For perovskite quantum dots, after stirring evenly, chloroform was removed by rotary evap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com