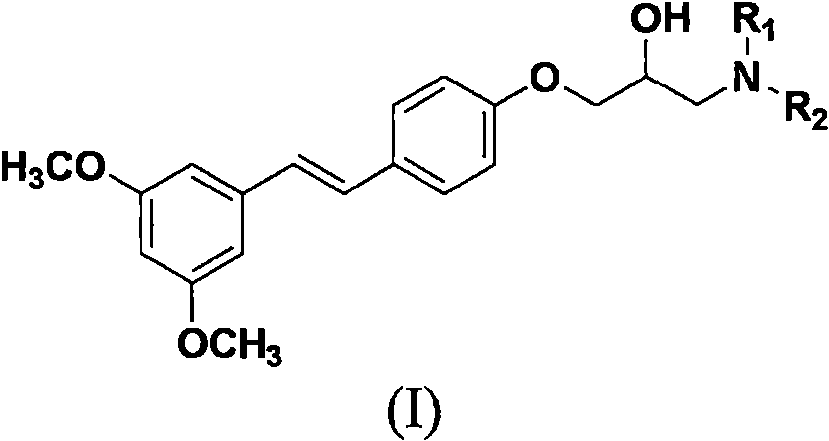
Pterostilbene amine compound containing isopropanol aromatic ether structure as well as preparation method and application of pterostilbene amine compound
A pterostilbene compound technology, applied in the field of pterostilbene compounds and their preparation, can solve the problems of endangering the safety of crops, increasing the resistance of plant pathogenic microorganisms, and affecting the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of pterostilbene epoxy intermediate 1
[0054] Add 1.0 mmol of pterostilbene and 1.2 mmol of potassium carbonate into a 25 mL round bottom flask containing 10 mL of anhydrous DMF, and stir at room temperature until pterostilbene is completely dissolved. Continue to drop 1.3mmoL of epibromohydrin, and stir at 35°C for 8 hours until complete reaction. The reaction was stopped, and 50 mL of ethyl acetate was added, and the organic layer was washed with saturated ammonium chloride and saturated brine, dried over anhydrous sodium sulfate, precipitated, and subjected to column chromatography to obtain a white solid with a yield of 88.5%. Its NMR data are: 1 H NMR (400MHz, CDCl 3 )δ7.44 (d, J=8.7Hz, 2H, phenyl-H), 7.04 (d, J=16.3Hz, 1H, phenyl-C H =CH), 6.91 (dd, J=12.5, 3.7Hz, 3H, phenyl-CH=C H &phenyl-H), 6.66 (d, J=2.2Hz, 2H, phenyl-H), 6.39 (t, J=2.2Hz, 1H, phenyl-H), 4.23 (dd, J=11.0, 3.0Hz, 1H, 1 / 2phenyl-O-CH 2 ), 3.94 (dd, J=11.0, 5.7...
Embodiment 2
[0055] Example 2: (E)-1-amino-3-(4-(3,5-dimethoxystyryl)phenoxy)propan-2-ol
[0056] Add 1.0mmoL of pterostilbene epoxy intermediate 1 and 1.1mmoL of potassium carbonate into a round-bottomed flask containing 5mL of isopropanol, stir at room temperature until intermediate 1 is completely dissolved, then add 2.0mmoL of ammonia water dropwise, and heat to 60 After reacting at ℃ for 6 hours, the reaction was stopped, the solvent was removed by distillation under reduced pressure, and column chromatography gave a white solid with a yield of 85.1%.
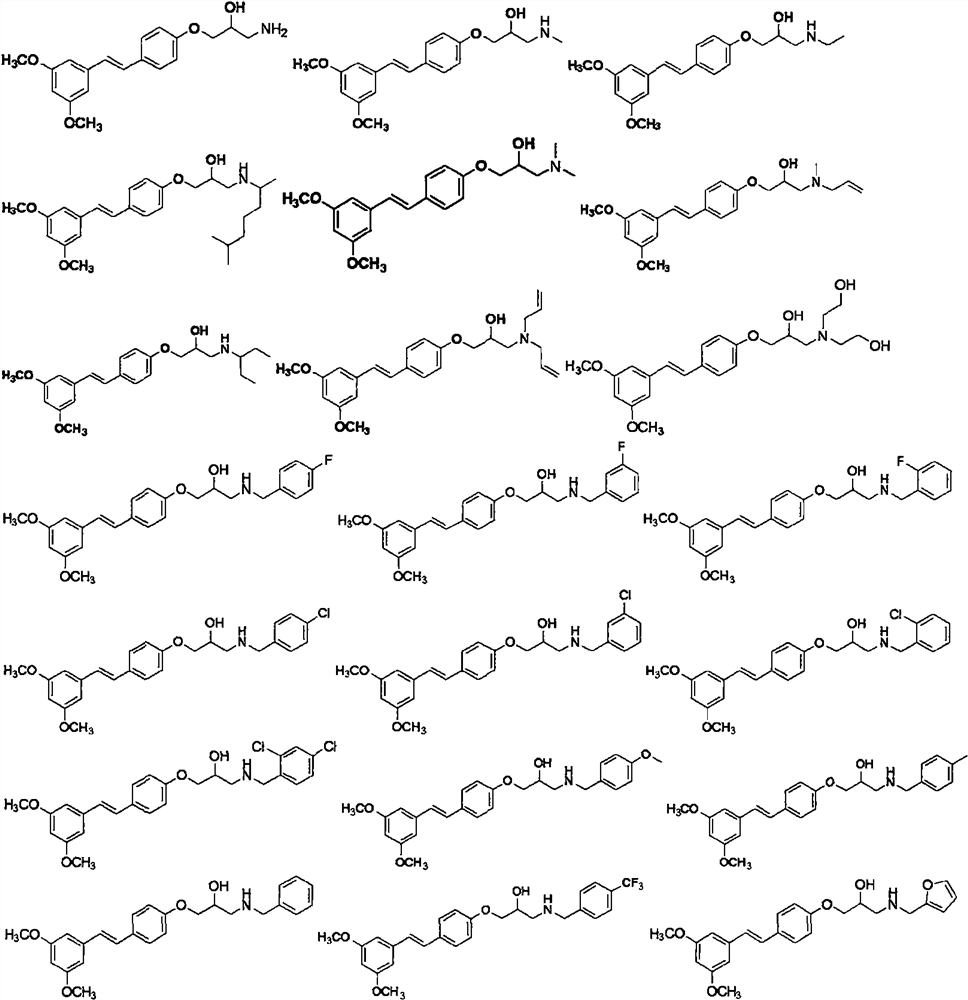
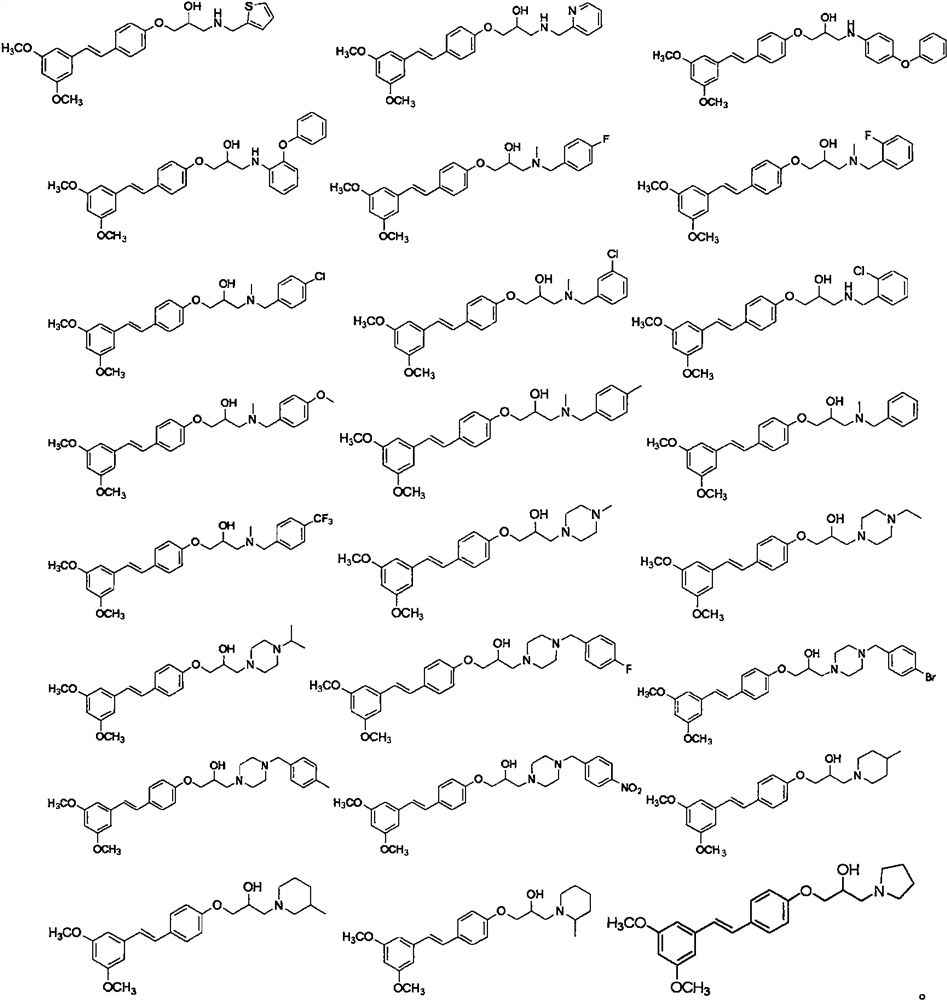
[0057] Other target compounds were synthesized according to the steps of Examples 1 and 2 using corresponding raw materials or substituents. The structure, H-NMR and C-NMR data of the synthesized pterostilbene compounds containing isopropanol aromatic ether structure are shown in Table 1, and the physicochemical properties are shown in Table 2.
[0058] H NMR spectrum, C NMR data and high resolution mass spectrum data of the compounds...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com