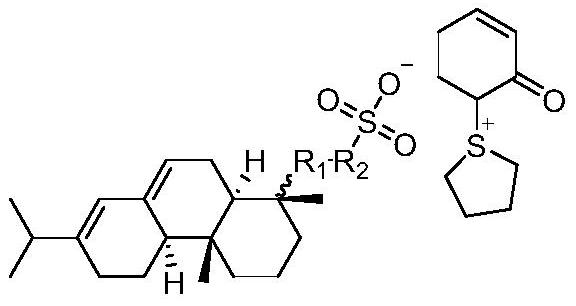
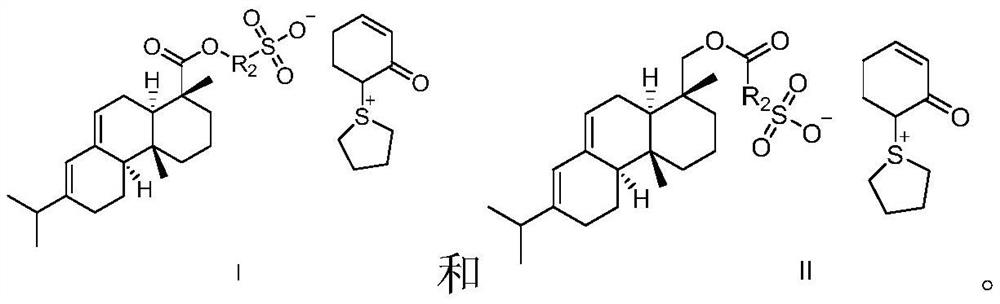
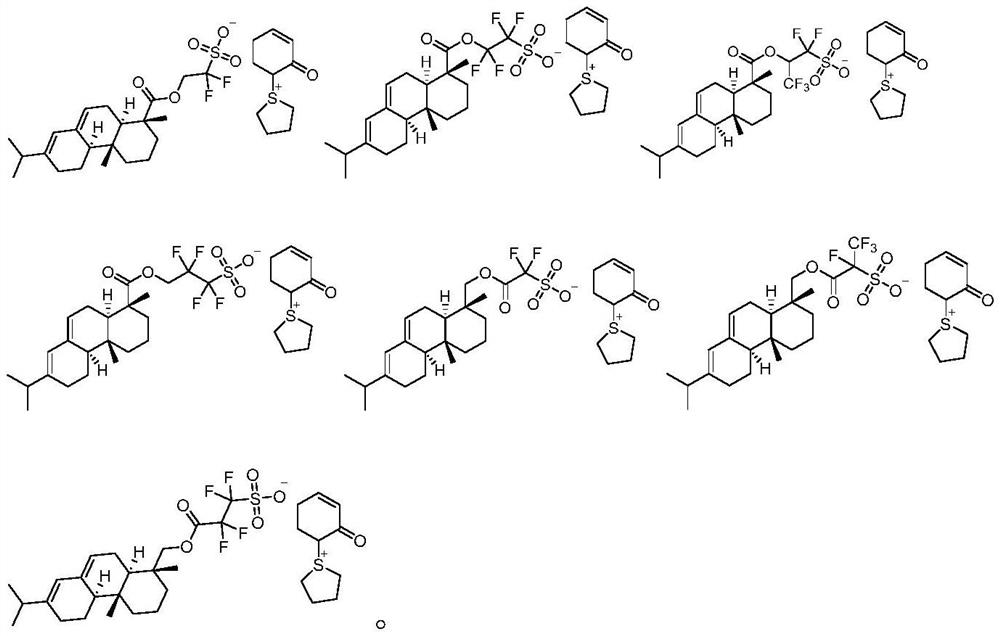
Sulfonium sulfonate photoacid generator synthesized from abietic acid and synthesis method of sulfonium sulfonate photoacid generator
A technology of photoacid generator and synthesis method, which is applied in the field of photoresist, can solve the problems of exposure influence, poor transparency, etc., and achieve the effects of excellent etching resistance, uniform dissolution, and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] The first step: Abietic acid 1-1 (10g, 33.1mmol), 1,1-difluoro-2-hydroxyl ethane-1-sodium sulfonate (6.1g, 33.1mmol) was added in toluene (100g), Add p-toluenesulfonic acid (0.8g, 4.6mmol), the reaction solution was heated to reflux for 16 hours, the reaction solution was cooled and filtered to obtain a solid, the solid was washed three times with acetonitrile (100g×3), the mixed acetonitrile solution was concentrated, and added to Beat in methyl tert-butyl ether (100 g), filter the above mixed solution, collect and dry the filter cake to obtain solid compound 1-2 (12.5 g, 26.7 mmol, yield 80.6%);
[0040] The second step: under the protection of nitrogen flow, (cyclohexa-1,5-dienyloxy)-trimethyl-silane (4.5g, 26.7mmol) and tetramethylene sulfoxide (2.8g, 26.9 mmol) solution in chloroform (250g), cooled to -30 degrees Celsius, slowly added trifluoroacetic anhydride (8g, 38.1mmol) within 30 minutes, stirred for 30 minutes, added 1-2 (12.5g, 26.7mmol) of satu...
Embodiment 2
[0042]
[0043] The first step: abietic acid 2-1 (10g, 33.1mmol), 1,1,2,2-tetrafluoro-3-hydroxypropane-1-sodium sulfonate (7.7g, 32.9mmol) and sulfuric acid (0.5g , 5.1 mmol) was added to toluene (150 g), heated to reflux for 18 hours and cooled to room temperature. The mixture was filtered to obtain a solid, which was washed three times with acetonitrile (100 g x 3). Concentrate the mixed acetonitrile solution, and add it into methyl tert-butyl ether (100g) for beating, filter the above mixed solution, collect and dry the filter cake to obtain intermediate 2-2 (13.5g, 26.0mmol, yield 78.7%).
[0044] The second step: under the protection of nitrogen flow, (cyclohexa-1,5-dienyloxy)-trimethyl-silane (4.4g, 26.1mmol) and tetramethylene sulfoxide (2.7g, 25.9 mmol) was dissolved in chloroform (200g), cooled to -30 degrees Celsius, slowly added trifluoroacetic anhydride (6.5g, 30.9mmol) within 30 minutes, stirred for 30 minutes, and added 2-2 (13.5g , 26.0mmol) of saturated a...
Embodiment 3
[0046]
[0047]The first step: abietic acid (10g, 33.1mmol) is dissolved in anhydrous tetrahydrofuran (100g), cooled to 0 degrees Celsius with an ice-water bath, under the protection of a nitrogen stream, slowly add lithium aluminum hydride (2.5g, 65.9mmol), The reaction solution was heated to reflux for 12 hours, the reaction solution was cooled to 0 degrees Celsius, 2.5 g of water was added, and 15% sodium hydroxide solution (6 g) was slowly added dropwise, then 7.5 g of water was added, raised to room temperature and stirred for half an hour, and 50 g of tetrahydrofuran was added to dilute , filtered with a funnel equipped with diatomaceous earth, the filter cake was washed with tetrahydrofuran (50g), the filtrate was dried over anhydrous sodium sulfate, and concentrated under vacuum to obtain intermediate 3-2 (9.1g, 31.5mmol, yield: 95.4% ).
[0048] The second step: join intermediate 3-2 (9.1g, 31.5mmol), sodium carboxydifluoromethanesulfonate (6.2g, 31.3mmol) in tolue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com