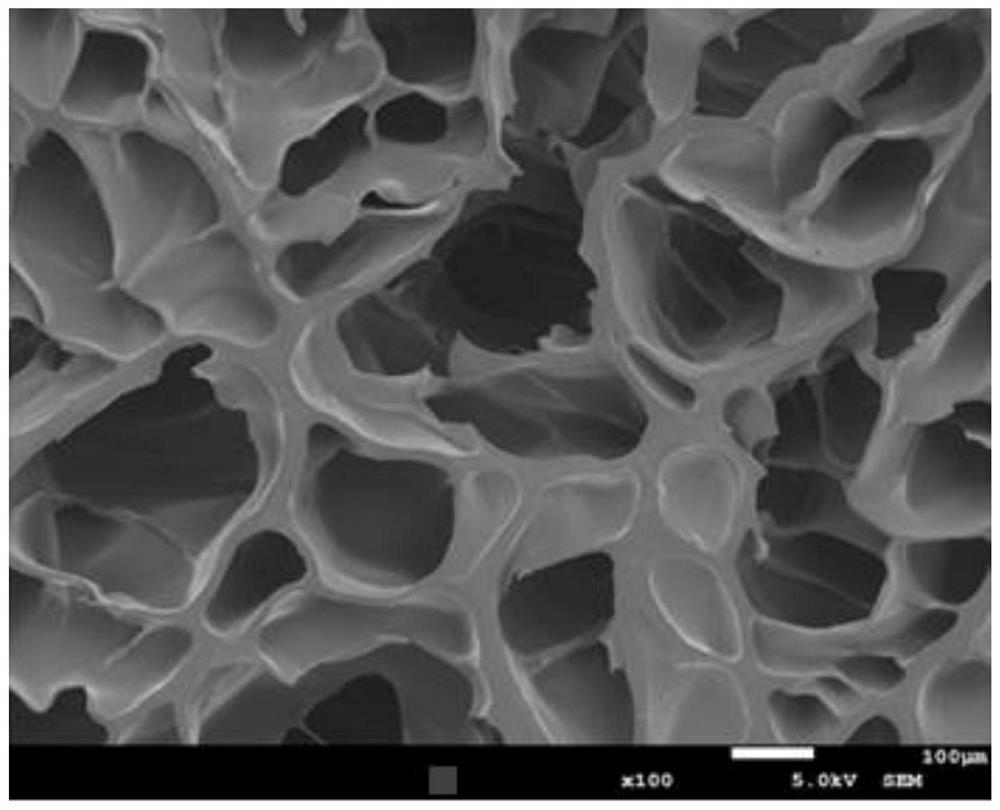
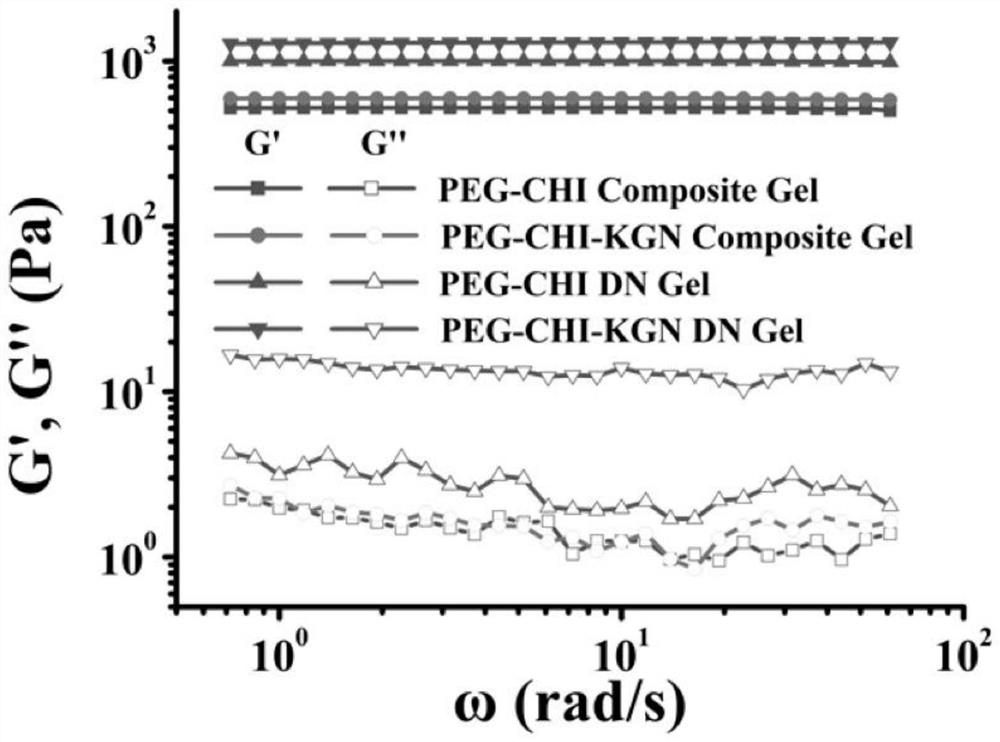
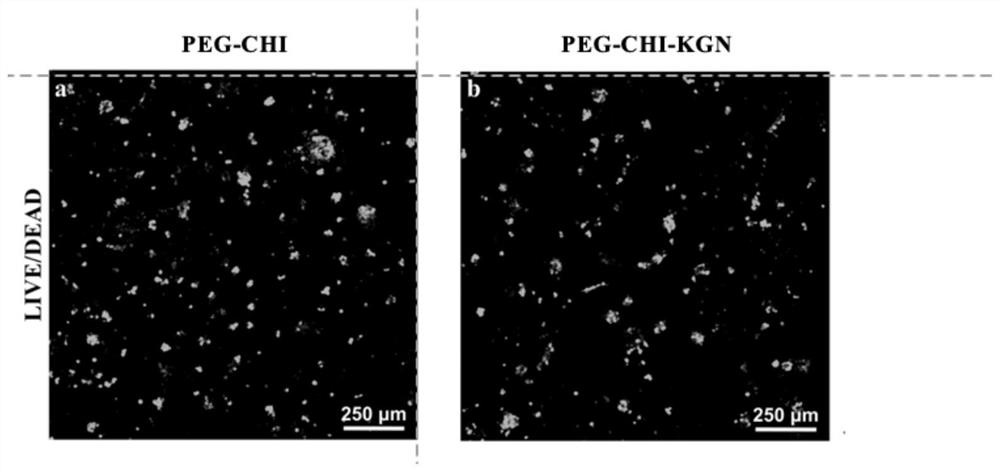
Functionalized double-network hydrogel as well as preparation method and application thereof
A dual-network, hydrogel technology, applied in medical science, prosthesis, tissue regeneration, etc., can solve the problems of poor stability and tissue selection specificity, limited cell deposition and distribution, short biological half-life, etc., and achieves simple preparation process Convenience, good cell affinity, effect of good microstructure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] In the second aspect, the present invention provides a method for preparing a functionalized double network hydrogel, which can be used to prepare the functionalized double network hydrogel provided in the first aspect of the present invention, the preparation method includes the following step:
[0067] (1) Chitosan is mixed and reacted with KGN activated by EDC / NHS to obtain a chitosan-KGN compound;
[0068] (2) the chitosan-KGN compound that step (1) obtains and Tetra-PEG-NH 2 Prepared as a first precursor solution;
[0069] (3) Tetra-PEG-NHS is formulated into the second precursor solution;
[0070] (4) Mixing the first precursor solution and the second precursor solution and then standing still to form a gel to obtain a composite hydrogel, and then subjecting the composite hydrogel to activation treatment to obtain a functional double network hydrogel.
[0071] Step (1): Step (1) is the step of coupling KGN to chitosan (CHI) by chemical means to obtain a CHI-KGN...
Embodiment 1
[0096] Preparation of CHI-KGN compounds
[0097] A CHI-KGN compound is prepared by using carbodiimide chemistry to form a chemically connected amide bond between the KGN terminal carboxyl group and the CHI amine group.
[0098] The preparation method comprises the following steps:
[0099] Weigh KGN 120mg, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) 726mg, N-hydroxysuccinimide (NHS) 434.8mg and dissolve in 20mL distilled water , adjust the pH value of the solution to 5.7, stir and react at 37° C. for 2 h, fully activate the carboxyl group of KGN, and obtain an activated KGN solution.
[0100] Weigh 3600 mg of chitosan and add it to the above-mentioned activated KGN solution, then add distilled water to make the total volume of the solution reach 150 mL, and stir and react at 37° C. for 24 h.
[0101] The reactant after the reaction was dialyzed in a dialysis bag with a molecular weight cut-off of 20 kD, and the distilled water was changed every 12 hours...
Embodiment 2
[0110] Preparation of CHI-KGN compounds
[0111] A CHI-KGN compound is prepared by using carbodiimide chemistry to form a chemically connected amide bond between the KGN terminal carboxyl group and the CHI amine group.
[0112] The preparation method comprises the following steps:
[0113] Weigh KGN 120mg, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) 726mg, N-hydroxysuccinimide (NHS) 434.8mg and dissolve in 20mL distilled water , adjust the pH value of the solution to 5.7, stir and react at 37° C. for 2 h, fully activate the carboxyl group of KGN, and obtain an activated KGN solution.
[0114] Weigh 4800 mg of chitosan and add it to the above-mentioned activated KGN solution, then add distilled water to make the total volume of the solution reach 150 mL, and stir and react at 37° C. for 24 h.
[0115] The reactant after the reaction was dialyzed in a dialysis bag with a molecular weight cut-off of 20 kD, and the distilled water was changed every 12 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com