Method for determining glutaraldehyde in water based on metal organic framework material composite substrate surface enhanced Raman spectroscopy
A surface-enhanced Raman, metal-organic framework technology, applied in the field of chemical detection, can solve the problems of complex structure, difficult synthesis, and complicated operation of fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
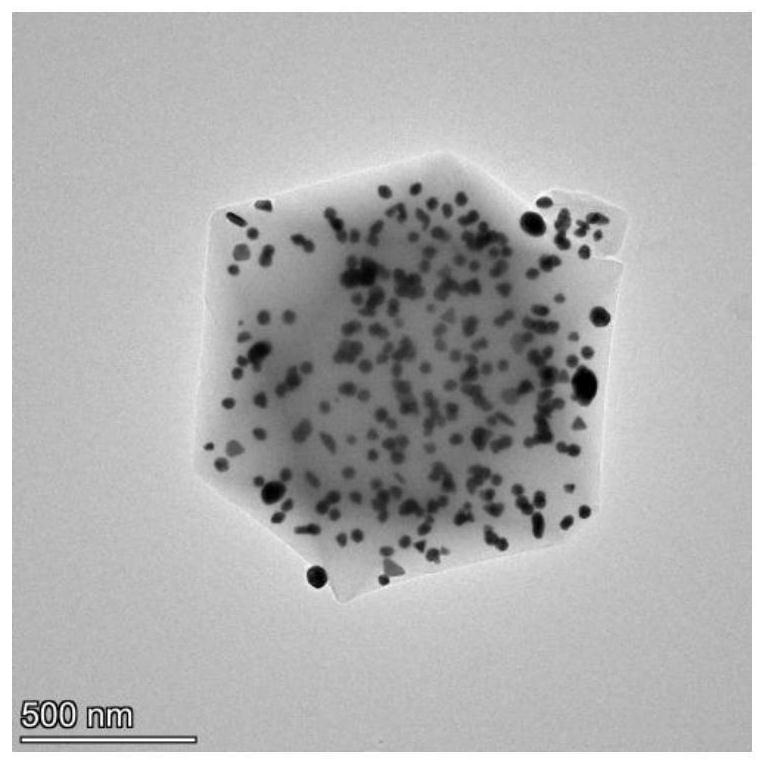
[0040] The present invention provides gold doped MIL-101(Cr). In the present invention, the preparation method of the gold-doped MIL-101(Cr) preferably comprises: dispersing MIL-101(Cr) in an aqueous solution of chloroauric acid, then mixing it with sodium citrate, and performing a redox reaction to obtain Gold doped MIL-101(Cr).
[0041] In the present invention, preferably, MIL-101 (Cr) is dispersed in an aqueous chloroauric acid solution to obtain an aqueous dispersion of MIL-101 (Cr) and chloroauric acid.
[0042] In the present invention, there is no special limitation on the dispersion operation of the MIL-101(Cr), and the operation of solid-liquid dispersion well-known to those skilled in the art can be used. In the present invention, the dispersion of MIL-101(Cr) preferably includes ultrasonication and stirring performed in sequence. In the present invention, there is no special limitation on the power of the ultrasound, and the conventional ultrasound power of those...
Embodiment 1
[0087] (1) Provide gold-doped MIL-101(Cr) (hereinafter referred to as Au@MIL-101)
[0088] ① Preparation of MIL-101(Cr) by hydrothermal method:
[0089] 1.1620g terephthalic acid, 2.8000g Cr(NO 3 ) 3 9H 2 O, 33.6g of ultrapure water, 0.4166mL of 40% hydrofluoric acid (0.4909g in mass) were homogenized by ultrasound, and then hydrothermally reacted at 220°C for 8 hours in a 50mL closed reactor. After cooling the hydrothermal product to room temperature, centrifuge at 3000rpm for 3min to obtain the crude product; then reflux the obtained crude product in ethanol at 80°C for 5h, and after cooling to room temperature, centrifuge at 3000rpm for 3min to obtain the preliminary Purified crude product; reflux the preliminary purified crude product in 200mL of 30mM ammonium fluoride solution at 60°C for 10h to obtain further purified crude product; After centrifugation for 3 minutes, the centrifuged solid was washed with ultrapure water at 60°C to remove ammonium fluoride, dried in ...
Embodiment 2
[0103] Repeat the method of embodiment 1, difference with embodiment 1 is as follows:
[0104] (1) Take eight 2mL centrifuge tubes in step (3), and add 30 μL of Au@MIL-101 / PATP with a concentration of 10 g / L, 2 μL of glacial acetic acid with a concentration of 4 mol / L and 30 μL of Glutaraldehyde solution.
[0105] (2) The medical wastewater sample was used as the sample solution to be tested.
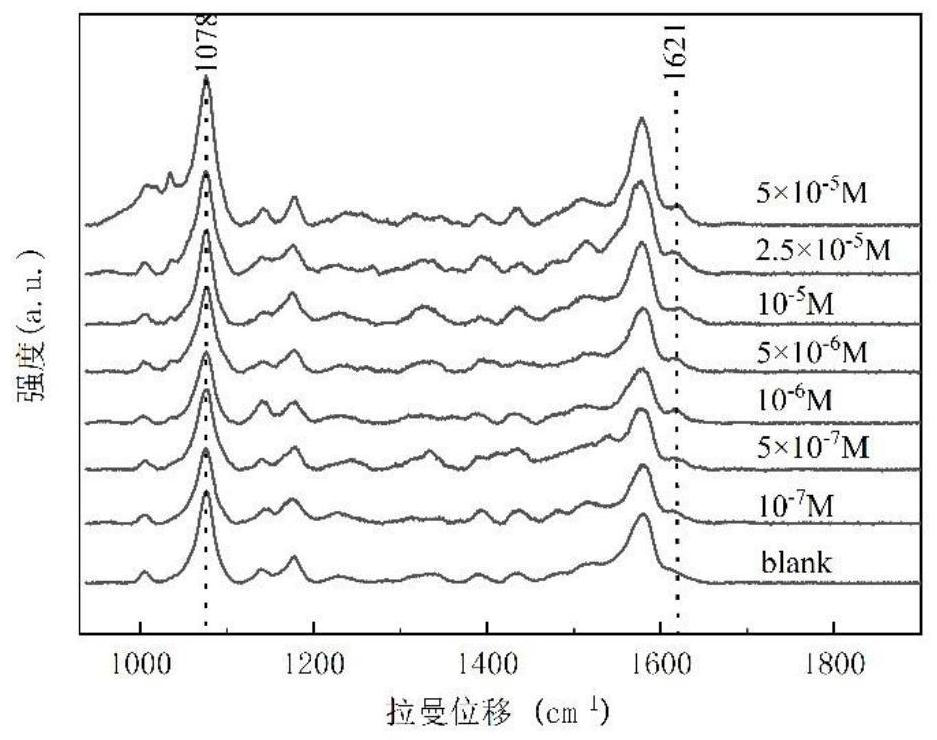
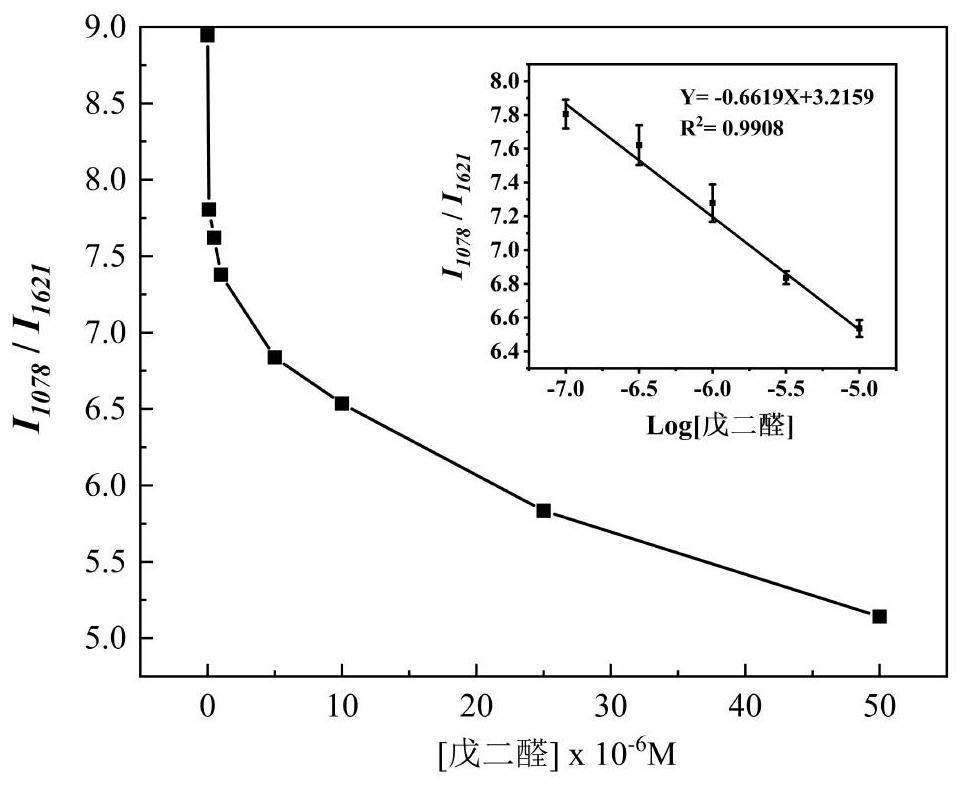
[0106] Then read 1078cm with the Raman spectrum of the solution to be tested -1 with 1621cm -1 The ratio of the peak height of the absorption peak at 1078 / I 1621 , obtain the concentration C of glutaraldehyde in the sample solution to be tested according to the working curve that embodiment 1 step (3) draws 样 , and then according to the formula ω=C 样 × M × 1000, calculate the content ω of glutaraldehyde in medical wastewater, and measure 5 sets of parallel data of the solution to be tested. The calculation results are shown in Table 1.
[0107] Embodiment 1~2 test solution laser...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass volume concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com