COVID-19 antigen detection card as well as preparation method and application thereof
A COVID-19, antigen detection technology, applied in the field of nanomaterials and nanomedicine, can solve the problems of rapid diagnosis and screening of patients with unfavorable pneumonia, long time and cycle of viral nucleic acid detection, and high requirements for hardware equipment, and achieves stable and stable labeled products. The effect of wide detection range and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The rare earth nanoprobe of this embodiment is sodium yttrium fluoride coated with sodium erbium fluoride with a core-shell structure, and has a particle size of 20nm to 30nm. Its composition is:
[0064] NaErF 4 @NaYF 4 , where NaErF 4 It is an all-erbium-doped core structure; NaYF 4 is the shell, @ means NaYF 4 Coated in NaErF 4 surface.
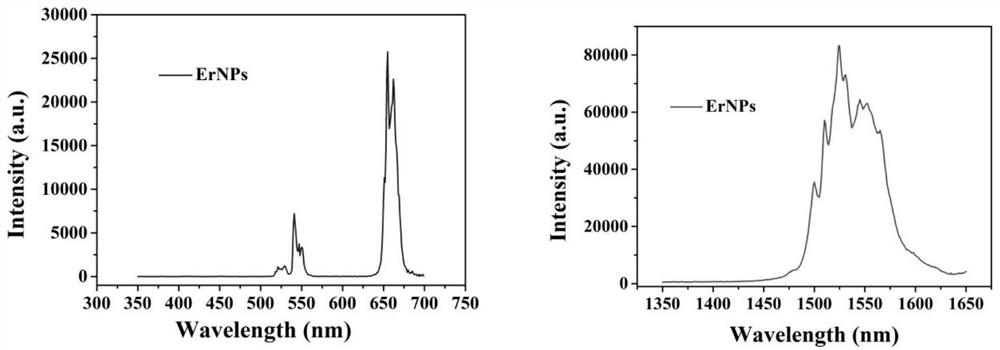
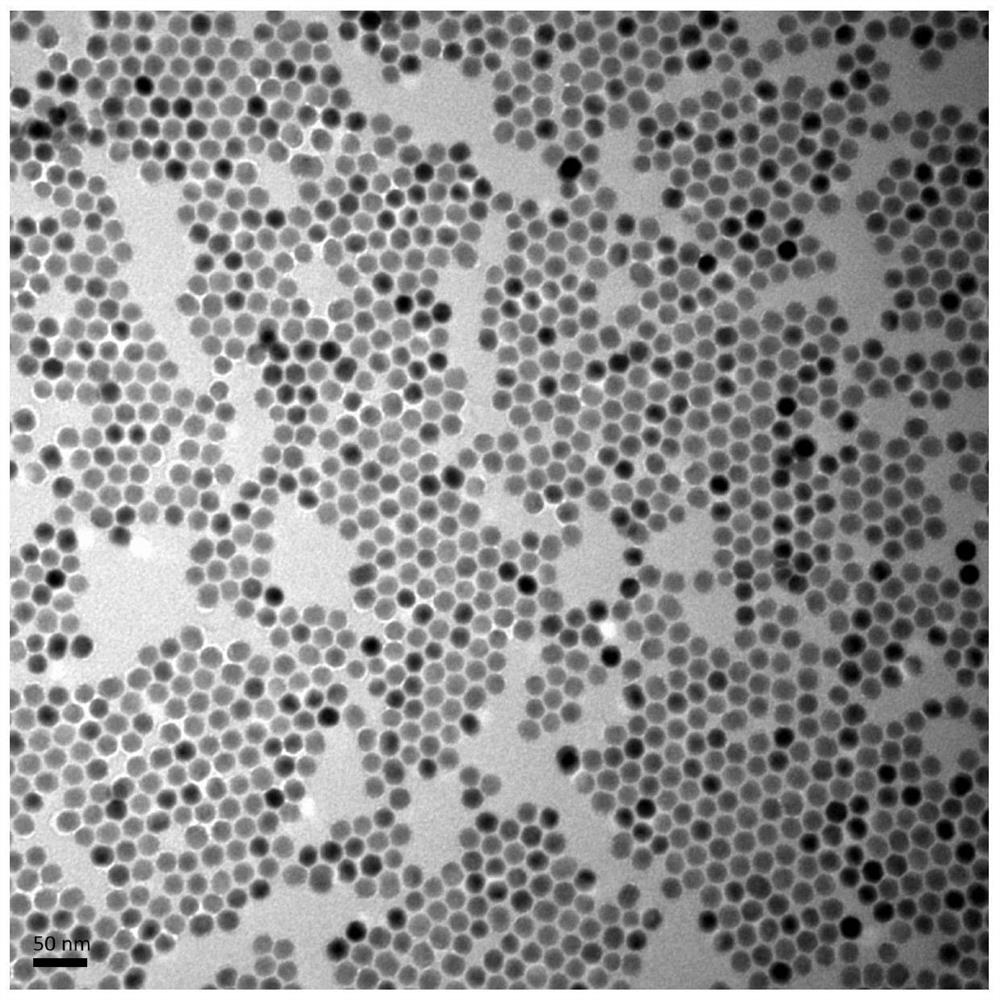
[0065] Further, the rare earth nanoprobe is stable in the ground state, and emits three emission fluorescences in the wavelength ranges of 500-550nm, 640-680nm and 1500-1600nm under the action of an excitation light source of 808nm. NaErF 4 @NaYF 4 Fluorescence spectra of rare earth nanoprobes excited at 808nm, such as figure 2 shown. NaErF 4 @NaYF 4 TEM images of rare earth nanoprobes, such as image 3 shown.
[0066] The method for preparing the rare earth nanoprobe of this embodiment comprises the following steps:
[0067] Step 1: Synthesis of erbium sodium fluoride core structure: In the container, add oleic acid ...
Embodiment 2
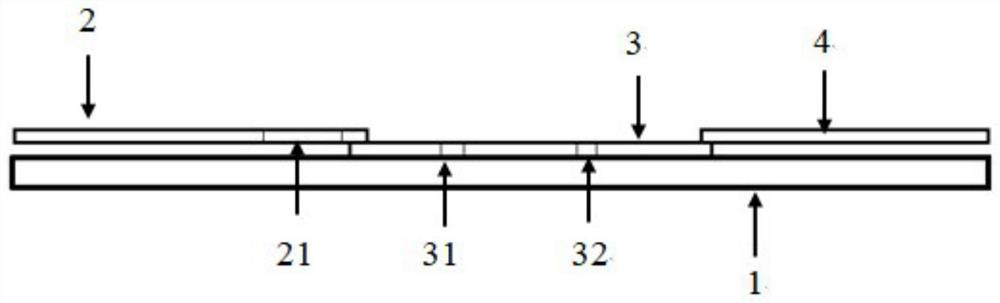
[0071] Such as figure 1 As shown, the COVID-19 antigen detection card of the present embodiment includes:
[0072] Substrate 1; as the substrate of the COVID-19 antigen detection card;
[0073] Coating film 2; arranged in the middle of the upper surface of the substrate 1;
[0074] Sample pad 3: overlapped at one end of the upper surface of the coating film 3
[0075] Absorbent paper 4; overlapped on the other end of the upper surface of the coating film 3;
[0076] Wherein, the sample pad 2 is sprayed with a microsphere thread 21, and the microsphere thread 21 is the COVID-19 monoclonal antibody 1 labeled with the rare earth nanoprobe provided in Example 1; the coating film 3 is provided with a detection line 31 and a quality control line 32, the detection line 31 is close to the sample pad 2, the detection line 31 and the quality control line 32 are parallel to each other, and the distance between them is 3-5mm; the detection line 31 is coated with COVID-19 monoclonal An...
Embodiment 3
[0087] The COVID-19 antigen detection kit of the present embodiment comprises:
[0088] COVID-19 Antigen Detection Kit includes Antigen Extraction Solution R1, which consists of 1% Triton X-100, 150mM Sodium Chloride, 1% Sodium Deoxycholate, 0.1% Sodium Lauryl Sulfate, Tris-Hcl (pH7.4).
[0089] COVID-19 antigen detection card: adopt the COVID-19 antigen detection card provided in Example 2;
[0090] ID card with calibration curve: The COVID-19 antigen detection card is used to measure different antigen concentration calibrators, with the antigen concentration as the abscissa and the fluorescence signal ratio as the ordinate, draw a standard curve, write and generate the corresponding QR code The information is stored in the ID card. The corresponding two-dimensional code information on the reagent card can be read and the corresponding antigen concentration can be measured by the dry fluorescent immunoassay analyzer.
[0091] The method for the quantitative detection of CO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com