Bio-based 2, 5-furandicarboxylic acid copolyester fiber as well as preparation method and application thereof
A technology of furandicarboxylic acid-based copolyester and furandicarboxylic acid, which is applied in the field of fibers, can solve the problems of low dyeing rate and lack of normal pressure dyeing, and achieve extended application range, excellent normal pressure dyeability, high The effect of dye uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Another aspect of the embodiments of the present invention also provides the preparation method of the aforementioned bio-based 2,5-furandicarboxylate copolyester fiber, which includes:
[0038] Under a protective atmosphere, make containing 2,5-furandicarboxylic acid, terephthalic acid, ethylene glycol, C 12 -C 20 A mixed reaction system of glycol ether composition, esterification catalyst, polycondensation catalyst, stabilizer and antioxidant is reacted to prepare 2,5-furandicarboxylic acid-based copolyester;
[0039] Drying the obtained 2,5-furandicarboxylate copolyester so that at least the moisture content of the 2,5-furandicarboxylate copolyester is below 70ppm;
[0040] And, spinning the dried 2,5-furandicarboxylic acid-based copolyester to obtain 2,5-furandicarboxylic acid-based copolyester as-spun fibers, and then heat-drawing to obtain 2,5-furandicarboxylate copolyester fiber.
[0041] In some more specific embodiments, the preparation method includes: maki...
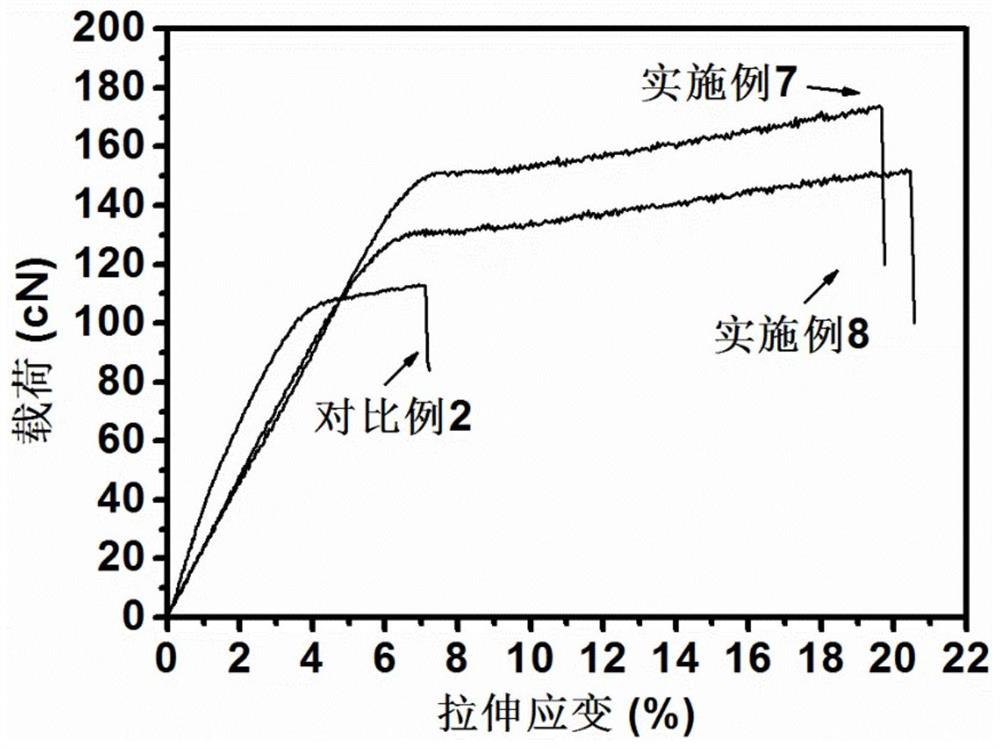
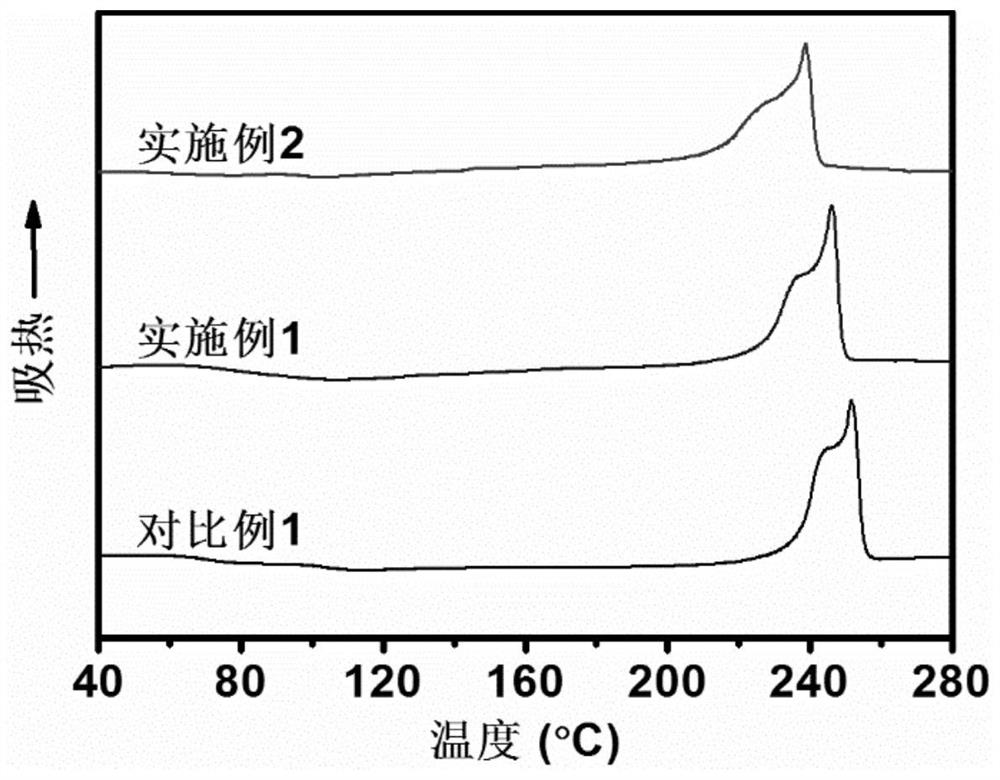
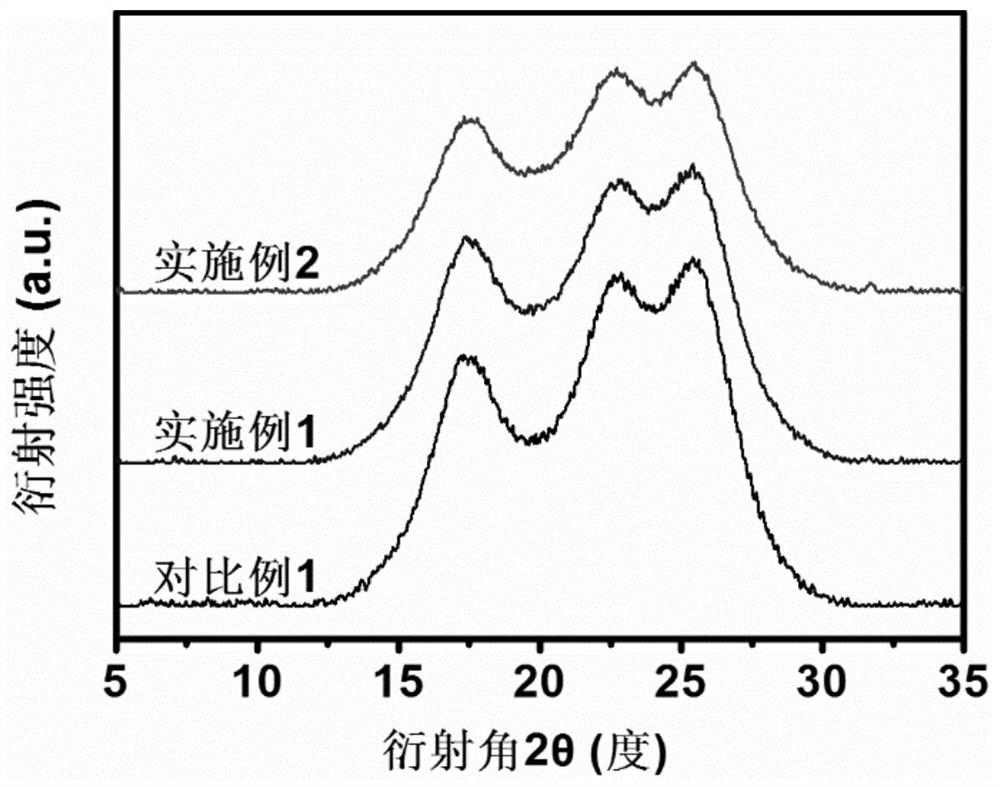
Embodiment 1
[0084] First, 2,5-furandicarboxylic acid (FDCA), terephthalic acid (PTA), ethylene glycol (EG), hexaethylene glycol and nonaethylene glycol composition (the molar ratio of the two is 1:1), anhydrous acetic acid Add zinc into the reactor according to the molar ratio of 0.01:0.99:1.6:0.0003:0.002, and gradually raise the temperature to 185°C under the protection of vacuum atmosphere and nitrogen for 3 hours; then add 0.12% of the total molar weight of FDCA and PTA into the reactor. Antimony trioxide, 0.20% triphenyl phosphate, 0.15% antioxidant-176, slowly evacuated to 0.03MPa, and pre-polymerized at 198°C for 1h; finally, gradually raised the temperature to 260°C, and continued to evacuate to React below 200Pa for 4h to obtain 2,5-furandicarboxylic acid-based copolyester. After testing, the intrinsic viscosity of copolyester is 0.70dL / g; Weight average molecular weight (M w ) and molecular weight distribution (DI) are 23700g / mol, 1.63 respectively; entanglement molecular weigh...
Embodiment 2
[0088] First, 2,5-furandicarboxylic acid (FDCA), terephthalic acid (PTA), ethylene glycol (EG), decaethylene glycol, and anhydrous zinc acetate are added to the reaction according to the molar ratio of 0.10:0.90:1.6:0.003:0.002 In the still, under the protection of vacuum atmosphere and nitrogen, the temperature was gradually raised to 185° C. and the reaction was stirred for 3 h; then 0.12% tetrabutyl titanate, 0.20% triphenyl phosphate, 0.15% antioxidant-1010, slowly evacuated to 0.03MPa, prepolymerized at 190°C for 1 hour; finally, gradually raised the temperature to 260°C, and continued to evacuate below 200Pa for 4 hours to obtain 2,5-furandicarboxylic acid Copolyester. After testing, the intrinsic viscosity of copolyester is 0.75dL / g; Weight average molecular weight (M w ) and molecular weight distribution (DI) are 27910g / mol, 1.55 respectively; entanglement molecular weight (M e ) is 1180 g / mol.
[0089] Continuously blow the copolyester with hot air at 130°C for 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com