Terphenyl macrocyclic compound based on biphenyl aromatic hydrocarbon and preparation method of terphenyl macrocyclic compound
A technology of macrocyclic compounds and biphenyl aromatic hydrocarbons, applied in the field of supramolecular macrocycles and their synthesis and preparation, can solve the problems of low yield of macrocyclic compounds, insufficient number of alkoxy groups, complex reactions, etc., and achieve low cost, The effect of high reaction efficiency and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
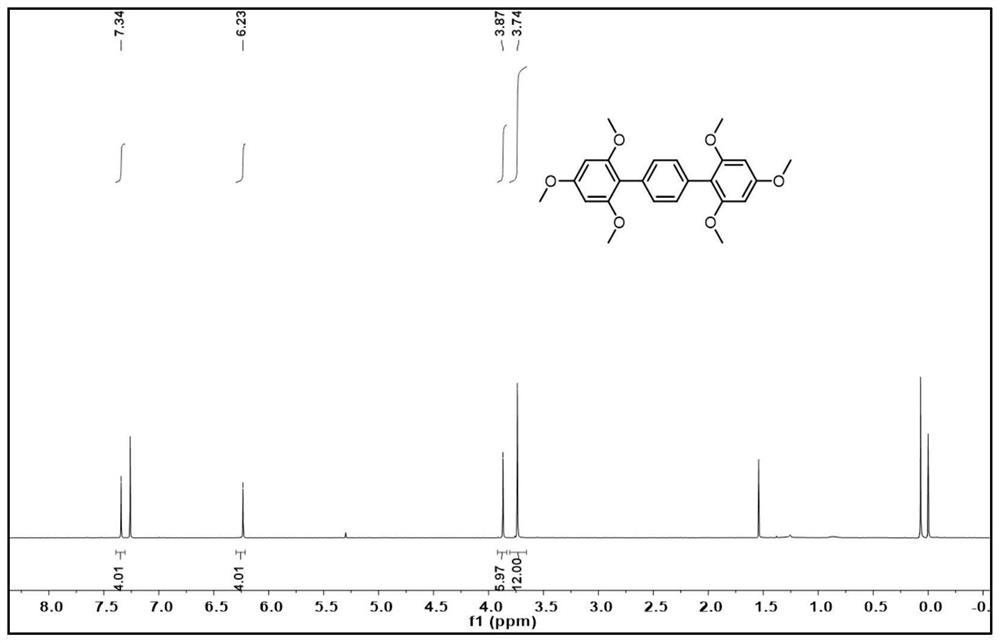
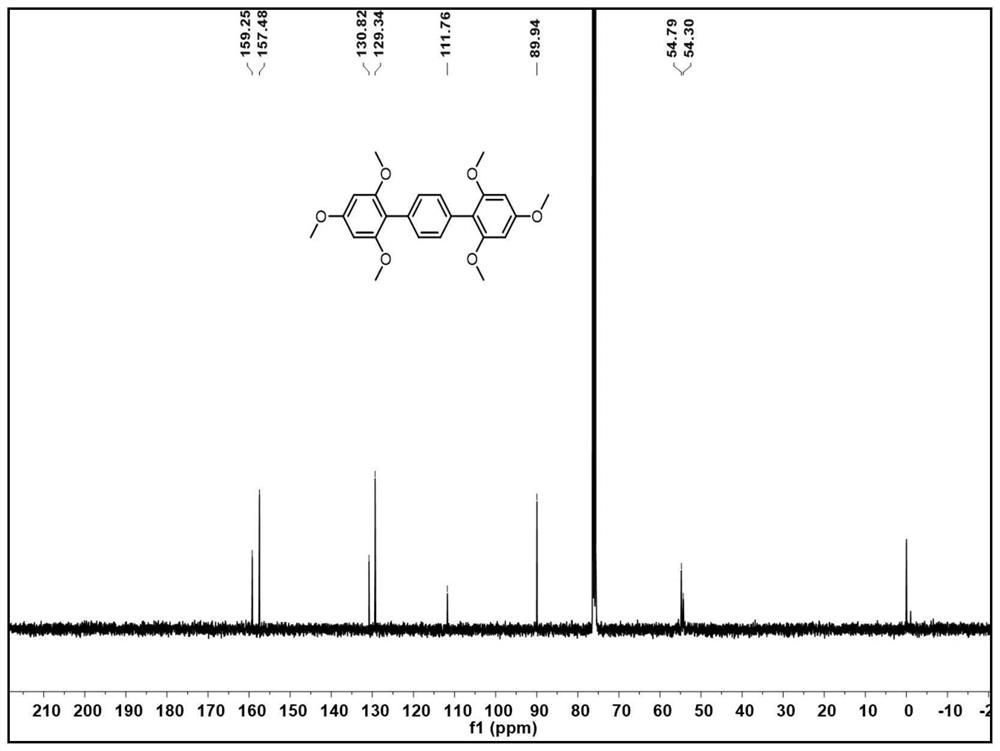
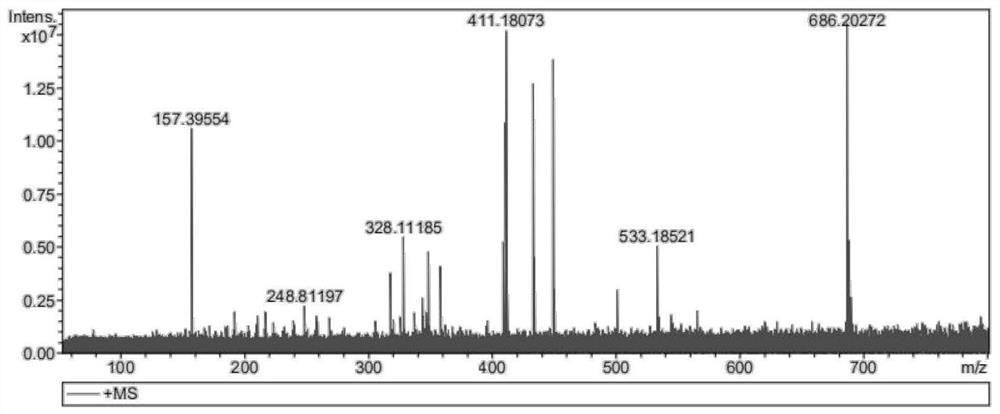
[0052] In this embodiment, a synthetic method of 2,2',4,4',6,6'--hexamethoxyterphenyl, the steps are as follows:
[0053] (1-1) In a 100ml round bottom flask, 1g of 1,4-dibromobenzene (CAS: 106-37-6) was completely dissolved in 30mL of 1,4-dioxane aqueous solution to obtain 1, 4-dibromobenzene mixed solution; the mass ratio of 1,4-dioxane and water in the 1,4-dioxane aqueous solution is 5:1;
[0054] (1-2) Then add 1.4g of 2,4,6-trimethoxyphenylboronic acid (CAS: 135159-25-0) and 0.4g of tetrakistriphenylphosphine palladium to the 1,4-dibromobenzene mixture , 4.2g sodium carbonate, obtain mixed liquid system;
[0055] (1-3) Then, the mixed liquid system was heated to 100° C. and refluxed overnight for reaction;
[0056] (1-4) When the reaction is over, cool the reaction solution to room temperature, spin the solvent to dry, dissolve in dichloromethane, extract with water three times, and wash the organic layer with anhydrous Na 2 SO 4 Dried, and spin-dried again to mix the...
Embodiment 2
[0060] This embodiment is basically the same as Embodiment 1, especially in that:
[0061] In this example, a synthetic method of 2,2',3,3'-tetramethoxyterphenyl, the steps are as follows:
[0062] (1-1) In a 100ml round bottom flask, 1g of 1,4-dibromobenzene (CAS: 106-37-6) was completely dissolved in 30mL of 1,4-dioxane aqueous solution to obtain 1, 4-dibromobenzene mixed solution; the mass ratio of 1,4-dioxane and water in the 1,4-dioxane aqueous solution is 5:1;
[0063] (1-2) Then add 1.2 g of 2,3-dimethoxyphenylboronic acid (CAS: 40972-86-9), 0.4 g of tetrakistriphenylphosphine palladium to the 1,4-dibromobenzene mixture, 4.2g sodium carbonate, obtain mixed liquid system;
[0064] (1-3) Then, the mixed liquid system was heated to 100° C. and refluxed overnight for reaction;
[0065] (1-4) When the reaction is over, cool the reaction solution to room temperature, spin the solvent to dry, dissolve in dichloromethane, extract with water three times, and wash the organic ...
Embodiment 3
[0069] This embodiment is basically the same as the previous embodiment, and the special features are:
[0070] In this example, a method for preparing a 2,2',4,4',6,6'-hexamethoxyterphenyl aromatic hydrocarbon macrocyclic compound, the steps are as follows:
[0071] (2-1) Add 0.1g monomer 2,2',4,4',6,6'-hexamethoxyterphenyl and 0.05g paraformaldehyde into a 100mL round bottom flask to obtain a mixed solution;
[0072] (2-2) Pour 60 mL of 1,2-dichloroethane into the mixed solution as a solvent, add 0.1 mL of boron trifluoride etherate complex while stirring, and monitor the reaction point;
[0073] (2-3) After 30 minutes of reaction, add 30 mL of saturated sodium bicarbonate solution to quench the reaction; then pour the reaction solution into a separatory funnel, wash with 50 mL of saturated sodium chloride solution, and dry over anhydrous sodium sulfate. The resulting mixture was separated with a silica gel column to obtain 60 mg of a white solid 2,2',4,4',6,6'-hexamethoxyt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com