Preparation method of 2, 3-dipicolinic acid
A technology of dipicolinic acid and concentrated sulfuric acid is applied in chemical instruments and methods, alkali metal oxides/hydroxides, inorganic chemistry, etc. Good adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
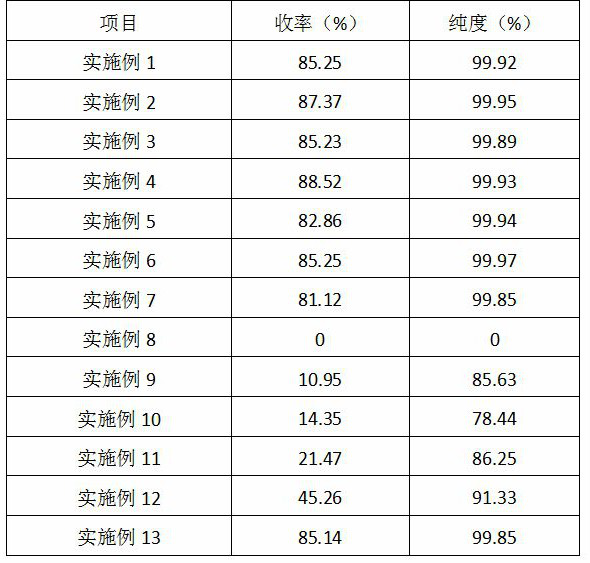
Examples
Embodiment 1
[0022] A kind of preparation method of 2,3-pyridinedicarboxylic acid, comprises the steps,
[0023] Step (1): Add 50g of quinoline with a mass fraction of 98%, 98g of concentrated sulfuric acid with a mass fraction of 98%, 160g of copper sulfate pentahydrate, and 92.8g of water into the oxidation reaction kettle, and continuously feed into the oxidation reaction kettle Oxygen, keep the pressure of the reactor at 4MPa, react at 70°C for 4h, obtain the precipitation of copper picolinate, and centrifuge to obtain filter cake A and mother liquor B;
[0024] Step (2): Mix 50g of water, the filter cake A, and 31g of sodium hydroxide into an alkali fusion kettle, react at 90°C for 1 hour, and filter while hot with a plate and frame filter press to obtain filter cake C and filtrate D ;
[0025] Step (3): Add the filtrate D into the neutralization and decolorization reaction kettle, add 8 g of non-magnetic ordinary activated carbon and stir for 20 minutes, and filter to obtain filter ...
Embodiment 2
[0028] Step (1): Evaporating and concentrating the mother liquor B obtained in Example 1, cooling the evaporated water vapor, crystals precipitated in the obtained concentrated solution K, and filtering to obtain a filter cake M and a filtrate N. The filtrate N, circulating water, 3g water, 50g mass fraction are 98% quinoline, 38g mass fraction are 98% concentrated sulfuric acid, 95g copper sulfate pentahydrate, continuously feed oxygen into the oxidation reactor to keep the reactor pressure It was 4MPa, and reacted at 70°C for 4h to obtain precipitation of copper pyridine acid salt, which was centrifuged to obtain filter cake A and mother liquor B.
[0029] Step (2): Mix 50g of water, the filter cake A, and 31g of sodium hydroxide into an alkali fusion kettle, react at 90°C for 1 hour, and filter while hot with a plate and frame filter press to obtain filter cake C and filtrate D ;
[0030] Step (3): Add the filtrate D into the neutralization and decolorization reaction kett...
Embodiment 3
[0033] The difference between Example 3 and Example 1 is that in step (1), oxygen is continuously fed into the oxidation reactor to keep the pressure of the reactor at 3 MPa, and the rest of the method for preparing the finished product of 2,3-pyridinedicarboxylic acid is the same as that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com