Alpha-monodeuterated amine compound, deuterated medicine and preparation method of same
A compound and synthetic method technology, applied in the preparation of organic compounds, aminohydroxy compounds, steroids, etc., can solve the problems of expensive reagents, poor regioselectivity, poor chemoselectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
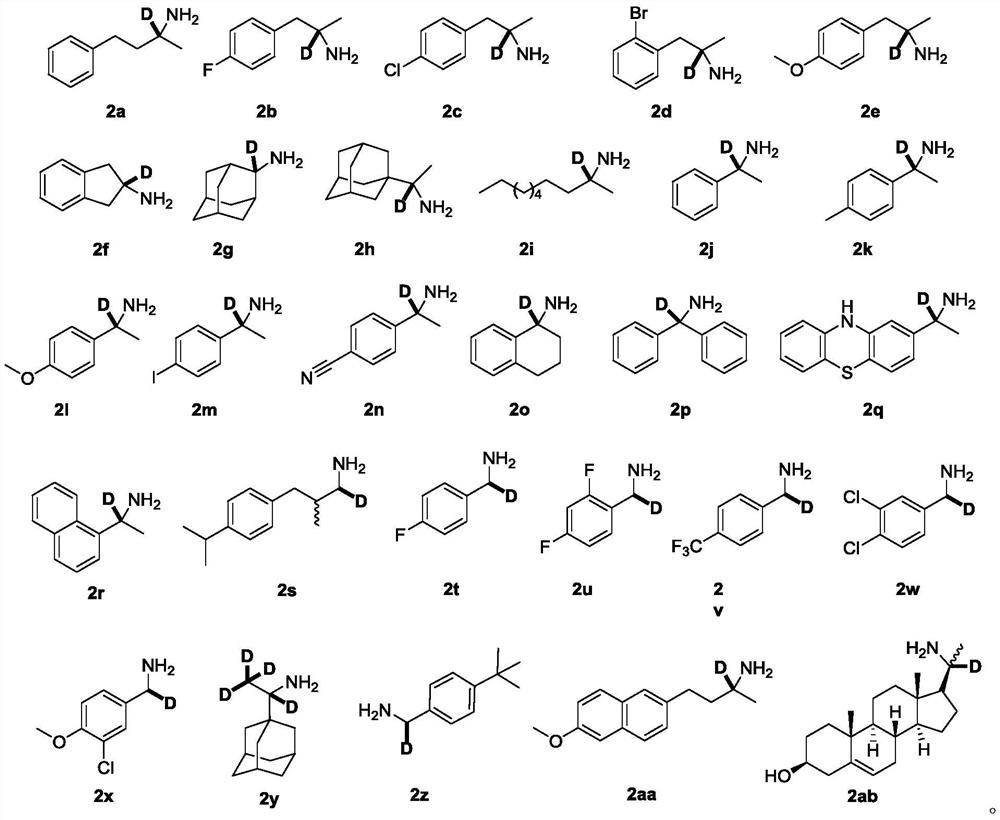
[0045] Into a 25 mL single-necked round bottom flask under the protection of argon, add samarium diiodide (SmI 2 ) solution (0.1mol / L) in tetrahydrofuran (0.1mol / L) 10mL, add heavy water 160mg (8.00mmol), compound 1a (32.6mg, 0.200mmol), and stir vigorously. The reaction mixture was stirred at room temperature for 15 min, after which time the reaction was quenched by bubbling air. Add ethyl acetate and 1mol / L sodium hydroxide solution for extraction, dry and concentrate the organic phase, add cyclopentyl methyl ether hydrochloride solution (3.0mol / L) to obtain 36.6 mg of the hydrochloride salt of target compound 2a, yield 98% , deuterium rate 96%.
[0046] The target product 2a obtained by the above synthesis method was detected by proton nuclear magnetic resonance spectrum and carbon spectrum, and the test results were as follows: 1 H NMR (300MHz, CDCl 3 )δ8.41(br,3H),7.28–7.14(m,5H),2.75(t,J=7.9Hz,2H),2.15(m,1H),1.92(m,1H),1.41(s,3H ); 13 C{ 1 H}NMR (75MHz, ...
Embodiment 2
[0048]
[0049] Into a 25 mL single-necked round bottom flask under the protection of argon, add samarium diiodide (SmI 2 ) in tetrahydrofuran (0.1mol / L) 14mL, add heavy water 224mg (11.2mmol), compound 1b (33.4mg, 0.200mmol), and stir vigorously. The reaction mixture was stirred at room temperature for 15 min, after which time the reaction was quenched by bubbling air. Add ethyl acetate and 1mol / L sodium hydroxide solution for extraction, dry and concentrate the organic phase, add cyclopentyl methyl ether hydrochloride solution (3.0mol / L) to obtain 32.4mg of the hydrochloride salt of target compound 2b, yield 85% , deuterium rate 96%.
[0050] The target product 2b obtained by the above synthesis method was detected by proton nuclear magnetic resonance spectrum and carbon spectrum, and the test results are as follows: 1 H NMR (300MHz, DMSO-d 6 )δ8.25(br,3H),7.29(m,2H),7.15(m,2H),3.03(d,J=13.3Hz, 1H),2.67(d,J=13.3Hz,1H),1.10( s,3H); 13 C{ 1 H}NMR (75MHz, DMSO-d 6 )δ16...
Embodiment 3
[0052]
[0053] Into a 25 mL single-necked round bottom flask under the protection of argon, add samarium diiodide (SmI 2 ) in tetrahydrofuran (0.1mol / L) 10mL, add heavy water 160mg (8.00mmol), compound 1c (36.7mg, 0.200mmol), and stir vigorously. The reaction mixture was stirred at room temperature for 15 min, after which time the reaction was quenched by bubbling air. Add ethyl acetate and 1mol / L sodium hydroxide solution for extraction, dry and concentrate the organic phase, add cyclopentyl methyl ether hydrochloride solution (3.0mol / L) to obtain 35.2mg of the hydrochloride salt of the target compound 2c, yield 85% , deuterium rate 96%.
[0054] The target product 2c obtained by the above synthesis method was detected by proton nuclear magnetic resonance spectrum and carbon spectrum, and the test results are as follows: 1 H NMR (300MHz, DMSO-d 6 )δ8.29(br,3H),7.38(m,2H),7.28(m,2H),3.05(d,J=13.3Hz,1H),2.69(d,J=13.3Hz,1H),1.10( s,3H); 13 C{ 1 H}NMR (75MHz, DMSO-d 6 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com