Monoclonal antibody drug oral nanogel and preparation method thereof
A nanogel and anti-drug technology, which is applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of unseen research reports, large molecular weight of drugs, and loss of biological activity, so as to improve encapsulation efficiency and increase compliance sex, guaranteed active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This example is to adopt the method provided by the present invention to prepare adalimumab oral nanogel.
[0032] Preparation steps:
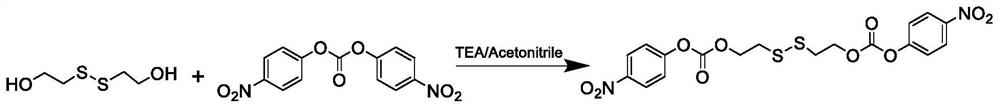
[0033] 1. Synthesis of cross-linking agent
[0034] Weigh an appropriate amount of 2-hydroxyethyl disulfide and bis(p-nitrophenyl)carbonate (the ratio of the amount of substances is 1:2.4), put them in a round bottom flask, add 10mL of anhydrous acetonitrile, stir to dissolve, and then Anhydrous triethylamine was added dropwise (the amount of the substance was twice that of 2-hydroxyethyl disulfide), and the reaction was stirred at room temperature for 24 hours. After the reaction, the reaction solution was rotatably evaporated under reduced pressure at 45°C, the solvent was completely spin-dried, an appropriate amount of dichloromethane was added, washed 3 times with pure water, dried over anhydrous sodium sulfate, concentrated, ethyl acetate:petroleum ether=1 :3 Precipitate and wash to no impurity, obtain pale yellow solid, after dr...
Embodiment 2
[0044] This example is the preparation of adalimumab oral nano-preparation by adopting the method provided by the present invention.
[0045] Preparation steps:
[0046] 1. Synthesis of cross-linking agent
[0047] With embodiment 1.
[0048] 2. Preparation of Nanogels
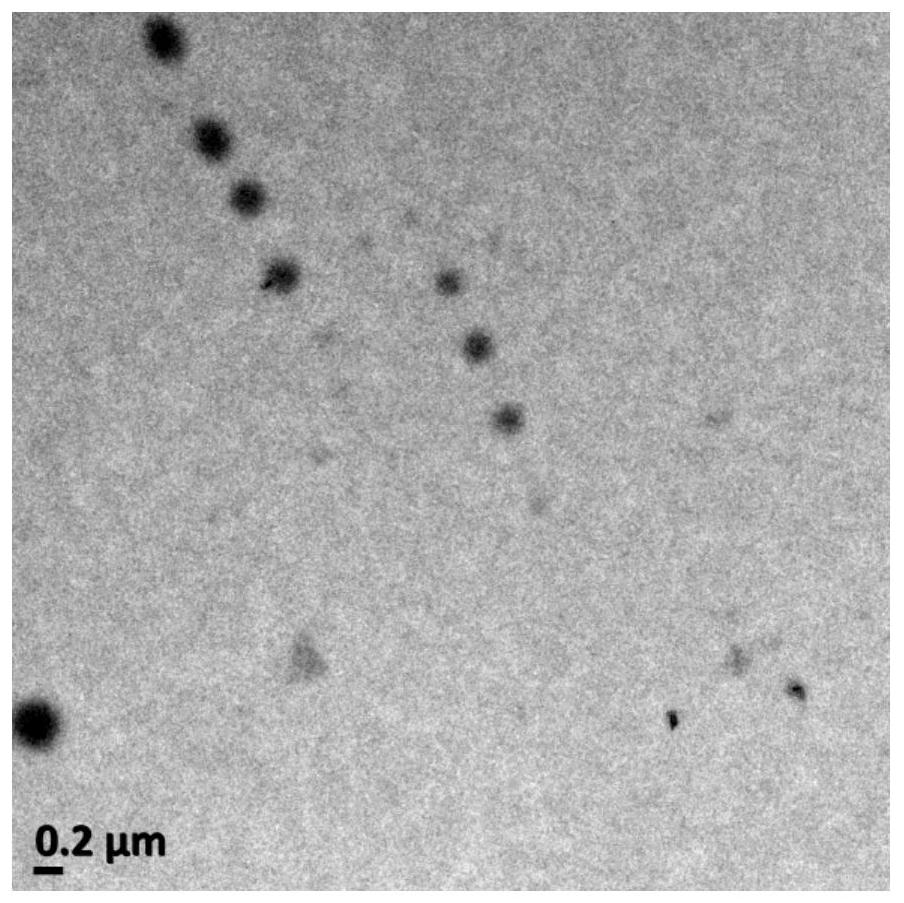
[0049] Weigh 1 mg of cross-linking agent and dissolve it in 1 mL of DMSO. Take 1 mL of adalimumab solution in PBS with a concentration of 0.08 μmol / mL, and adjust the pH value to 7.4. The DMSO solution of the cross-linking agent was slowly added dropwise to the antibody solution, and reacted at 25°C for 30 minutes. Take 1 mL of a PBS solution of hyaluronic acid with a concentration of 0.16 μmol / mL, add it dropwise to the above reaction solution, and continue the reaction at 25° C. for 30 minutes. The unreacted impurities were removed by dialysis, and finally the impurities were removed by using a Sephadex G100 Sephadex G100 column to obtain the adalimumab nanogel with a drug loading of 56.6% and an encaps...
Embodiment 3
[0051] This example is the preparation of adalimumab oral nano-preparation by adopting the method provided by the present invention.
[0052] Preparation steps:
[0053] 1. Synthesis of cross-linking agent
[0054] With embodiment 1.
[0055] 2. Preparation of Nanogels
[0056] Weigh 1 mg of cross-linking agent and dissolve it in 1 mL of DMSO. Take 1 mL of adalimumab solution in PBS with a concentration of 0.13 μmol / mL, and adjust the pH value to 7.4. The DMSO solution of the cross-linking agent was slowly added dropwise to the antibody solution, and reacted at 25°C for 30 minutes. Take 1 mL of a PBS solution of hyaluronic acid with a concentration of 0.26 μmol / mL, add it dropwise to the above reaction solution, and continue the reaction at 25° C. for 30 minutes. The unreacted impurities were removed by dialysis, and finally the impurities were removed by using a Sephadex G100 Sephadex G100 column to obtain the adalimumab nanogel with a drug loading capacity of 53.8% and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com