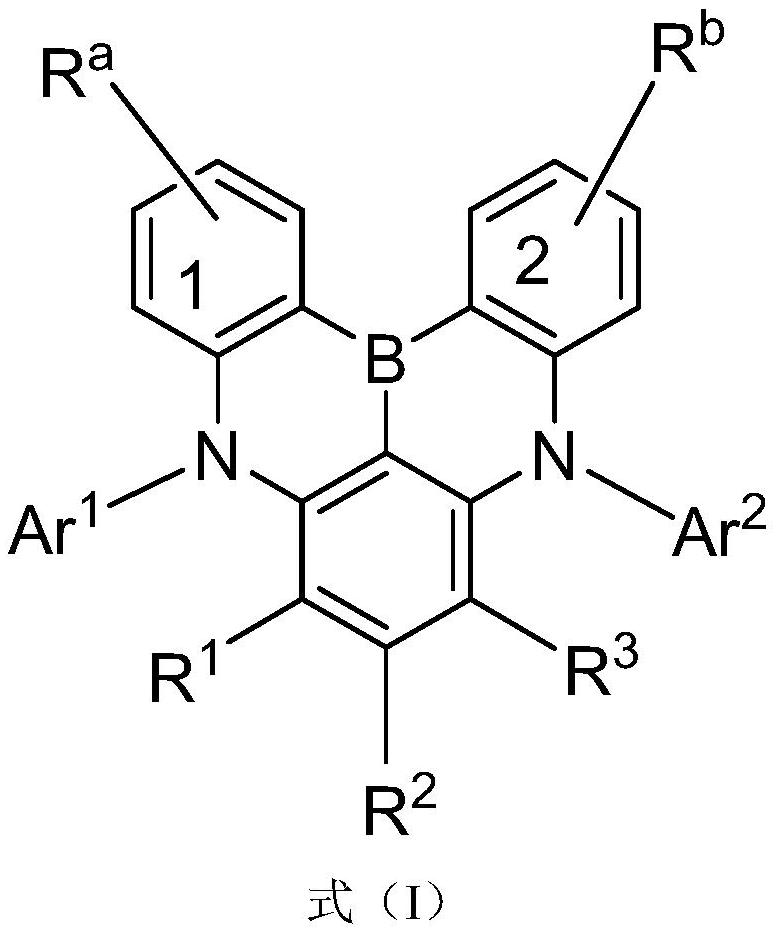
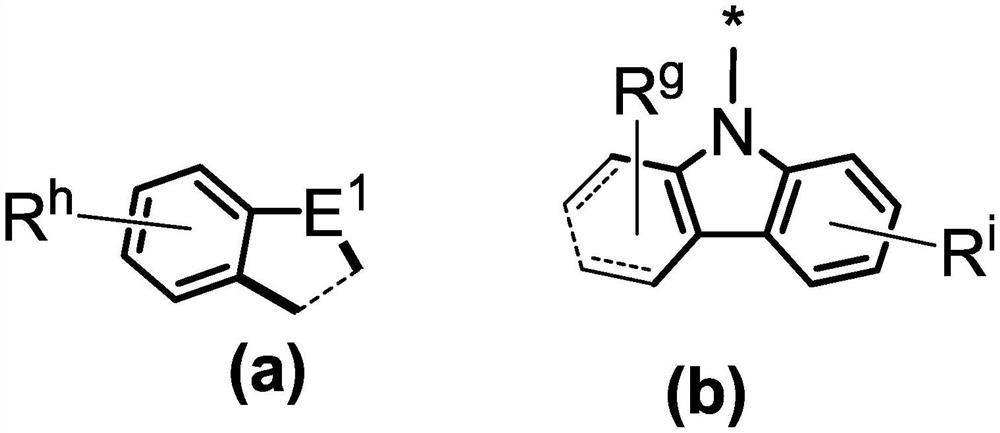
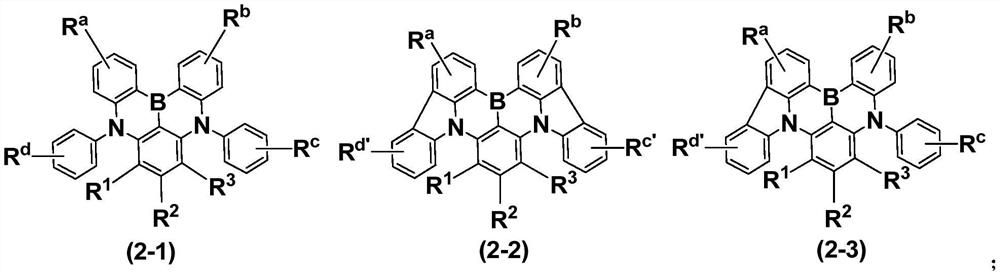
Compound and application thereof, and organic electroluminescent device containing compound
A compound and unsubstituted technology, applied in the field of organic electroluminescent devices, can solve the problems that the efficiency and life cannot meet the increasing requirements of display materials, and achieve the effect of reducing non-radiative transitions, reducing direct recombination, and increasing orbital energy levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0107] Synthesis of compound M17:
[0108]
[0109] (1) Preparation of compound M17-1:
[0110] Add 5,7-dihydro-5-phenylindolo[2,3-B]carbazole (33.2g, 100mmol), 1,3-dibromo-2-chloro-5 -Fluorobenzene (34.3g, 120mmol), cesium carbonate (39.1g, 150mmol), N,N-dimethylformamide (300ml), nitrogen replacement 3 times, 130°C heating reaction overnight. After the reaction stopped, after cooling to room temperature, 500ml of water was added and stirred for 10 minutes. A large amount of white solids were precipitated. After suction filtration, the filter cake was boiled and washed with ethanol for 2 hours, cooled and suction filtered to obtain 56.8g of white solid product with a yield of 95%. Molecular ion mass determined by mass spectrometry: 597.92 (theoretical value: 597.94).
[0111] (2) Preparation of compound M17-2
[0112] At room temperature, M17-1 (14.9g, 25mmol), bis(4-(methyl-d3))aniline (10.5g, 52mmol), Pd 2 (dba) 3(0.43g, 0.47mmol), s-Phos (0.38g, 0.94mmol), sodium t...
preparation example 11
[0116] Synthesis of Compound M21:
[0117]
[0118] (1) Preparation of compound M21-1:
[0119] Add 12H-benzofuro[2,3-a]carbazole (25.7g, 100mmol), 1,3-dibromo-2-chloro-5-fluorobenzene (34.3g, 120mmol ), cesium carbonate (39.1g, 150mmol), N,N-dimethylformamide (300ml), nitrogen replacement 3 times, 130 ℃ heating reaction overnight. After the reaction stopped, after cooling to room temperature, 500ml of water was added and stirred for 10 minutes. A large amount of white solids were precipitated. After suction filtration, the filter cake was boiled and washed with ethanol for 2 hours, cooled and suction filtered to obtain 48.6g of white solid product with a yield of 93%. Molecular ion mass determined by mass spectrometry: 522.94 (theoretical value: 522.90).
[0120] (2) Preparation of compound M21-2
[0121] At room temperature, M21-1 (26.1g, 50mmol), deuterated diphenylamine (8.7g, 50mmol), Pd 2 (dba) 3 (0.46g, 0.50mmol), s-Phos (0.38g, 0.92mmol), sodium tert-butoxide (...
Embodiment 1
[0127] This embodiment provides an organic electroluminescent device, the preparation process of which is as follows:
[0128] A glass plate coated with an ITO (thickness 150nm) transparent conductive layer is ultrasonically treated in a commercial cleaning agent, rinsed in deionized water, ultrasonically degreased in acetone:ethanol mixed solvent, and baked in a clean environment until the water is completely removed parts, cleaned with UV light and ozone, and bombarded with a beam of low-energy cations;
[0129] Place the above-mentioned glass substrate with the anode in a vacuum chamber, and evacuate to less than 1×10 -5 Pa, HI-2 and HT-4 were vacuum-deposited on the anode layer as the hole injection layer and the hole transport layer respectively, the evaporation rate was 0.1nm / s, and the evaporation film thickness was 10nm and 40nm respectively;
[0130] Vacuum-deposit "BFH-3:M1 (30nm, 3%wt)" on the hole transport layer as the light-emitting layer of the organic electrol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com