Canine parainfluenza virus attenuated strain, application thereof and vaccine
A technology of canine parainfluenza virus and attenuated strains, which is applied to veterinary vaccines, viruses, vaccines, etc., can solve the problems of high cost and weak pertinence, and achieve the effect of strong pertinence, low production cost and high virus price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
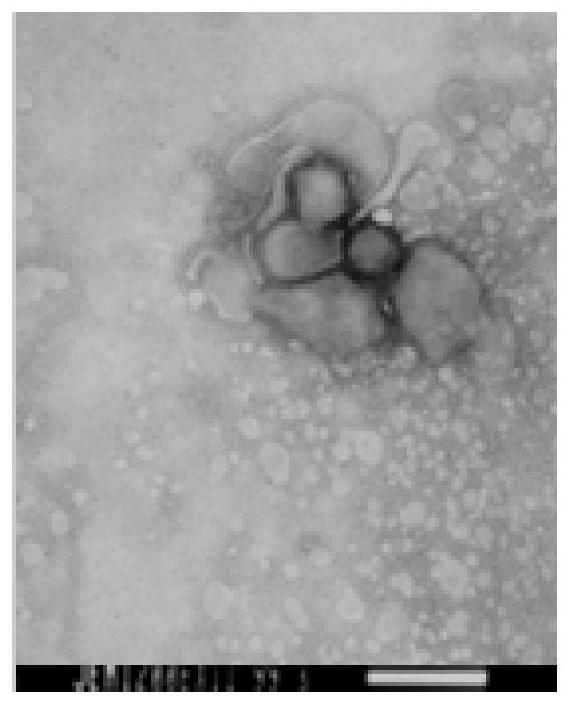
[0044] Example 1 Isolation and identification of natural attenuated CPIV / 15 strain of canine parainfluenza virus.
[0045] Materials and methods
[0046] Source of disease material: A puppy from Changchun Pet Hospital in Jilin Province had serous or mucopurulent rhinorrhea around the nostrils, accompanied by clinical symptoms such as mild cough, and was initially diagnosed as suspected canine parainfluenza virus disease. Collect five parts of nasal secretion disease materials (No. 1, No. 2, No. 3, No. 4, No. 5) in total, take their nasal secretions and add them into 3 times PBS buffer solution containing penicillin and streptomycin (pH 7.2) After repeated freezing and thawing for 3 times, centrifuge at 8000r / min at 4°C for 15min, filter and sterilize with a sterile filter (0.22μm), and store at -30°C after the bacterial test is negative.
[0047] Cells: Vero (F87) cell line was purchased from China Veterinary and Veterinary Drug Control Institute, and was preserved and suppli...
Embodiment 2
[0058] Example 2 Comprehensive Identification of Canine Parainfluenza Attenuated Strain CPIV / 15
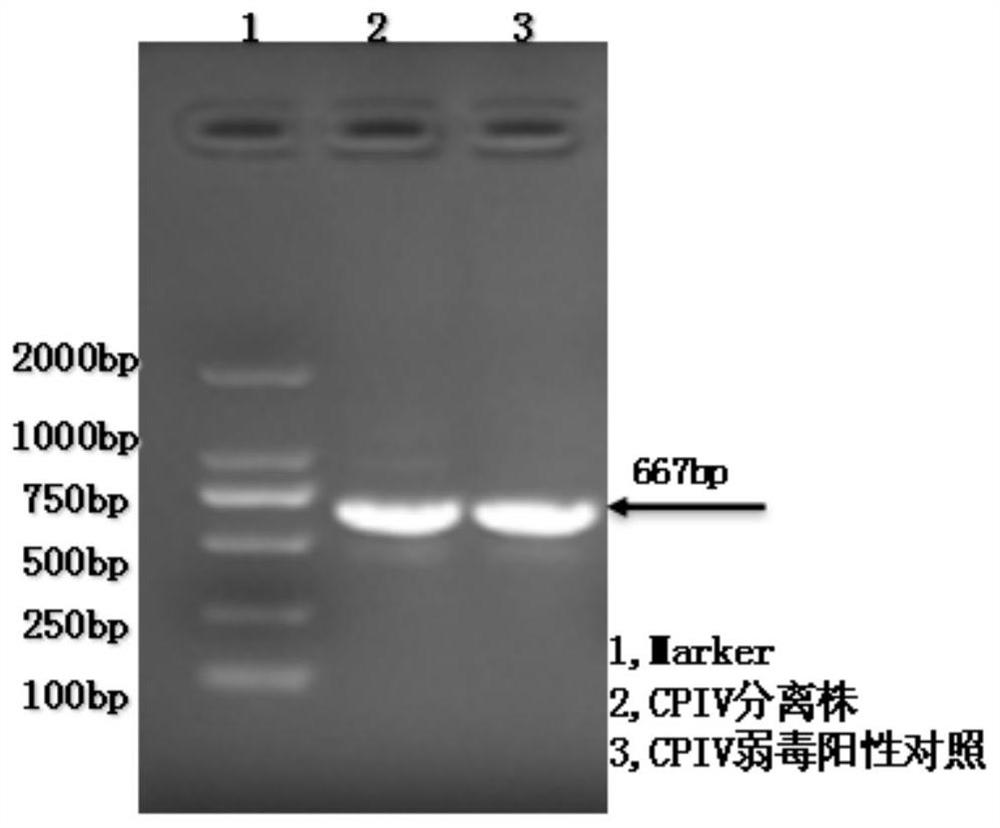
[0059] Viral nucleic acid type identification: 5-iododeoxyuridine (5-IUDR) method: culture Vero cells in a 96-well plate, and after the cells grow into a monolayer, pour out the growth medium and replace it with 2% fetal bovine serum DMEM maintenance solution, and 5-IUDR was added to the maintenance solution to make the final concentration 50 μg / mL, and then inoculate different dilutions of the virus solution in the cell culture wells, each dilution was repeated 4 times, and observed daily after inoculation Cytopathy. Take the negative logarithm of the highest dilution factor of the CPE of the virus as the titer of the virus, and determine the TCID of the virus growing on Vero cells 50 At the same time, the culture solution without 5-IUDR was used as a control. Because 5-IUDR has obvious inhibitory effect on DNA virus, it has no or only low inhibitory effect on RNA virus, and th...
Embodiment 3
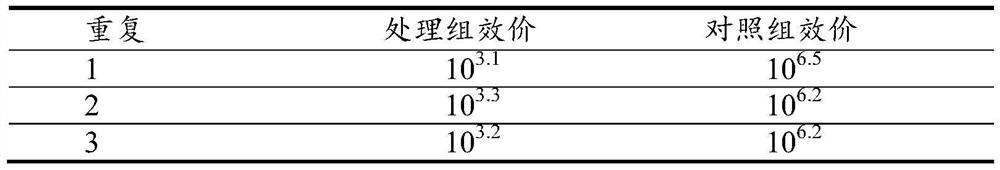
[0092] Example 3 Dog Body Passage Reversion Test
[0093] Test materials and methods:
[0094] Cells: African green monkey kidney passage cells (Vero), purchased from the China Veterinary Drug Administration and preserved by Beijing Sentaier Technology Co., Ltd.
[0095] Virus: canine parainfluenza virus attenuated CPIV / 15 strain, F50 generation; preserved and provided by Beijing Shengtaier Technology Co., Ltd.
[0096] Experimental animals: The dogs used in the experiment are 42-49 days old healthy beagle dogs with CPIV antibody SN negative or ≤2 that have not been immunized with canine parainfluenza virus vaccine.
[0097] Cell subculture stability test: use Vero subculture cells, adopt the method of synchronous poisoning, 37°C 5% CO 2 Cultivate under conditions, cultivate for 5 to 7 days and more than 80% of the cytopathic changes (CPE) will be harvested, and then continue to use Vero to culture and pass down to 60 generations (CPIV / 15F60) to observe the changes of each g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com